The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the coronavirus disease 2019 (COVID-19), is readily communicable between humans. Recent estimates indicate that COVID-19 has a mortality rate among symptomatic individuals of 2-8%.
The S protein is a target for most of the current SARS-CoV-2 vaccines that have been approved by the World Health Organization (WHO). Severe disease is prevented by these vaccines as a result of the induction of robust antibody and T-cell responses. SARS-CoV-2 vaccines have been shown to exhibit poorer responses in individuals with autoimmune conditions, potentially due to the vaccine being inhibited by immunosuppressant drugs.
In a recent study published on the preprint server bioRxiv*, researchers investigate the effects of five established immunosuppressant medications that target different pathways of the immune response. Moreover, the researchers were interested in determining whether the effects of these drugs could be regulated following changes to immunosuppressant regimens in mouse models. The authors then evaluated the immune response to SARS-CoV-2 S protein vaccinations in mice who had been administered immunosuppressant drugs.
Does immune suppression impair antibody responses to SARS-CoV-2 S protein?
Six groups of mice were selected, with five of the groups receiving immunosuppressant drugs and one control group receiving no drugs, to test the effect of these drugs on the effectiveness of SARS-CoV-2 vaccination. When compared to the control groups, all drug treatment groups displayed significant decreases in absorbances.
Following analysis of individual serum immunoglobulin G (IgG) and IgM levels, significant differences were observed between the groups, thus indicating that immunosuppressant drugs can impair antibody responses to SARS-CoV-2 S protein vaccines. To determine whether the IgG and IgM titer inhibition against the S protein would translate into functional deficiencies in anti-S response, the authors used a recombinant vesicular stomatitis virus (VSV), with the VSV glycoprotein replaced with the SARS-CoV-2 S protein to monitor the neutralization of the virus.
When comparing 50% neutralization activity 28 days post-primary vaccination, the sera from drug-treated mice were less potent when compared to the control group. This difference was not observed in mice that were administered leflunomide, which displayed no significant differences when compared to the control group.
The authors observed functional inhibition of antibody-mediated clearance of S protein-expressing cells among all drug-treated mice. Thus, this finding revealed that immunosuppressant drugs can inhibit vaccination responses against SARS-CoV-2.
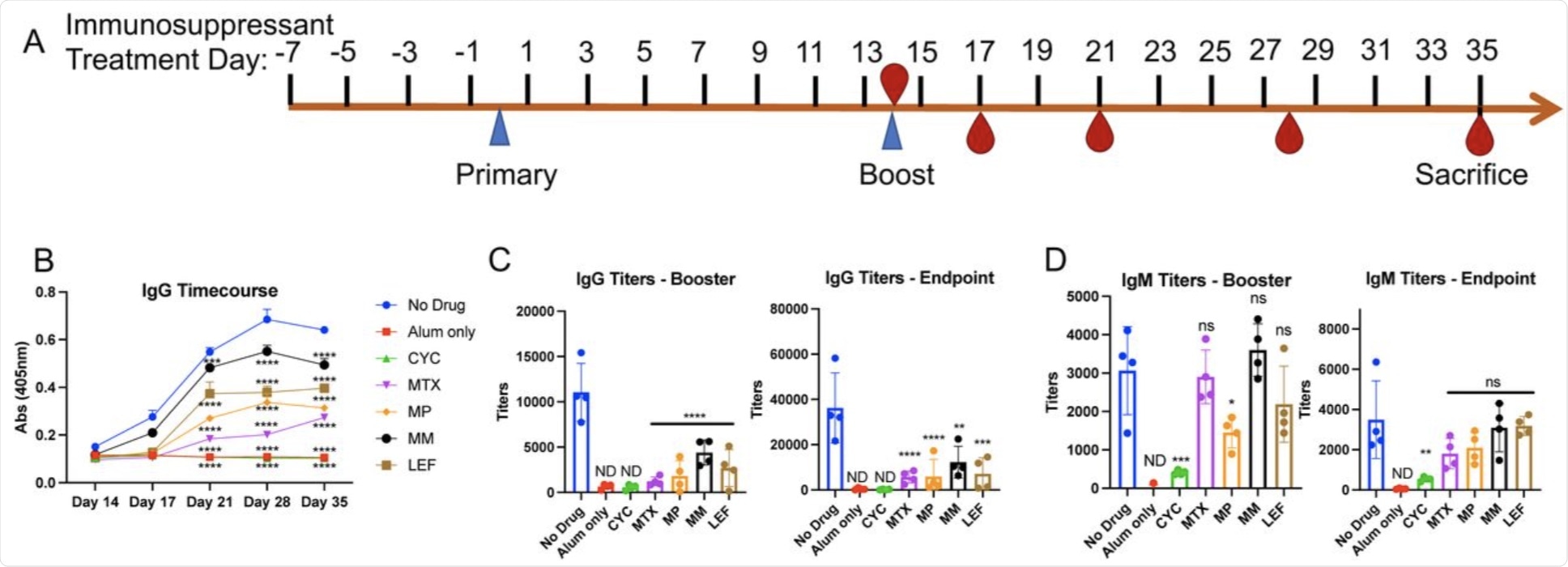 Changes in antibody levels to SARS-CoV-2 spike protein following immunosuppressant administration. A. Schematic of intraperitoneal SARS-CoV-2 spike protein immunization and immunosuppressant drug administration. Recombinant spike protein was injected at 0.5μg/50μl alum per mouse. Alum only mice received 50 μl of alum with no spike protein. Immunosuppressants were administered intraperitoneally at the indicated timepoints according to the concentrations in Supplementary Table 1. B. Serum was obtained from immunosuppressant-treated mice on days 14 (prior to booster immunization), 17, 21, 28, and 35 days (following booster immunization). ELISA was performed to determine serum IgG titers against spike protein. We did not detect IgG/IgM response to spike protein in the non-immunized mouse sera (alum only). Input was individual OD (405nm) values of sera at 1/3200 dilution and significance was determined for all time points following booster immunization. C. IgG and D. IgM titers after booster immunizations (day 21) and at endpoint (day 35). Averages of OD values at 405nm absorbance of technical replicates for individual mouse serum sample were used in regression curves to obtain titers (reciprocal serum dilutions) at absorbance of 0.5 at 405nm. Significance was determined as compared to No Drug control group. Alum only and CYC groups were non-detectable (ND).
Changes in antibody levels to SARS-CoV-2 spike protein following immunosuppressant administration. A. Schematic of intraperitoneal SARS-CoV-2 spike protein immunization and immunosuppressant drug administration. Recombinant spike protein was injected at 0.5μg/50μl alum per mouse. Alum only mice received 50 μl of alum with no spike protein. Immunosuppressants were administered intraperitoneally at the indicated timepoints according to the concentrations in Supplementary Table 1. B. Serum was obtained from immunosuppressant-treated mice on days 14 (prior to booster immunization), 17, 21, 28, and 35 days (following booster immunization). ELISA was performed to determine serum IgG titers against spike protein. We did not detect IgG/IgM response to spike protein in the non-immunized mouse sera (alum only). Input was individual OD (405nm) values of sera at 1/3200 dilution and significance was determined for all time points following booster immunization. C. IgG and D. IgM titers after booster immunizations (day 21) and at endpoint (day 35). Averages of OD values at 405nm absorbance of technical replicates for individual mouse serum sample were used in regression curves to obtain titers (reciprocal serum dilutions) at absorbance of 0.5 at 405nm. Significance was determined as compared to No Drug control group. Alum only and CYC groups were non-detectable (ND).
Can temporary suspension of immunosuppressant drugs recover antibody function against SARS-CoV-2?
To determine whether immunosuppressant regimen suspension would improve antibody response, the authors treated mice with cyclophosphamide (CYC), methotrexate (MTX), and methylprednisolone (MP), and then halted the treatment at the time of vaccination. The CYC treatment group of mice displayed negligible levels of IgG and IgM titers against the S protein, despite the treatment being interrupted one and three days before vaccination.
The mice who received MP displayed some restoration of antibody response when treatment was suspended before vaccination. Comparatively, the group which received MTX treatment showed significant increases in booster titer levels when treatment was halted. Depending on the drug in question, these results indicate that the antibody response to the SARS-CoV-2 S protein can be improved by modulating the immunosuppression treatment regimen.
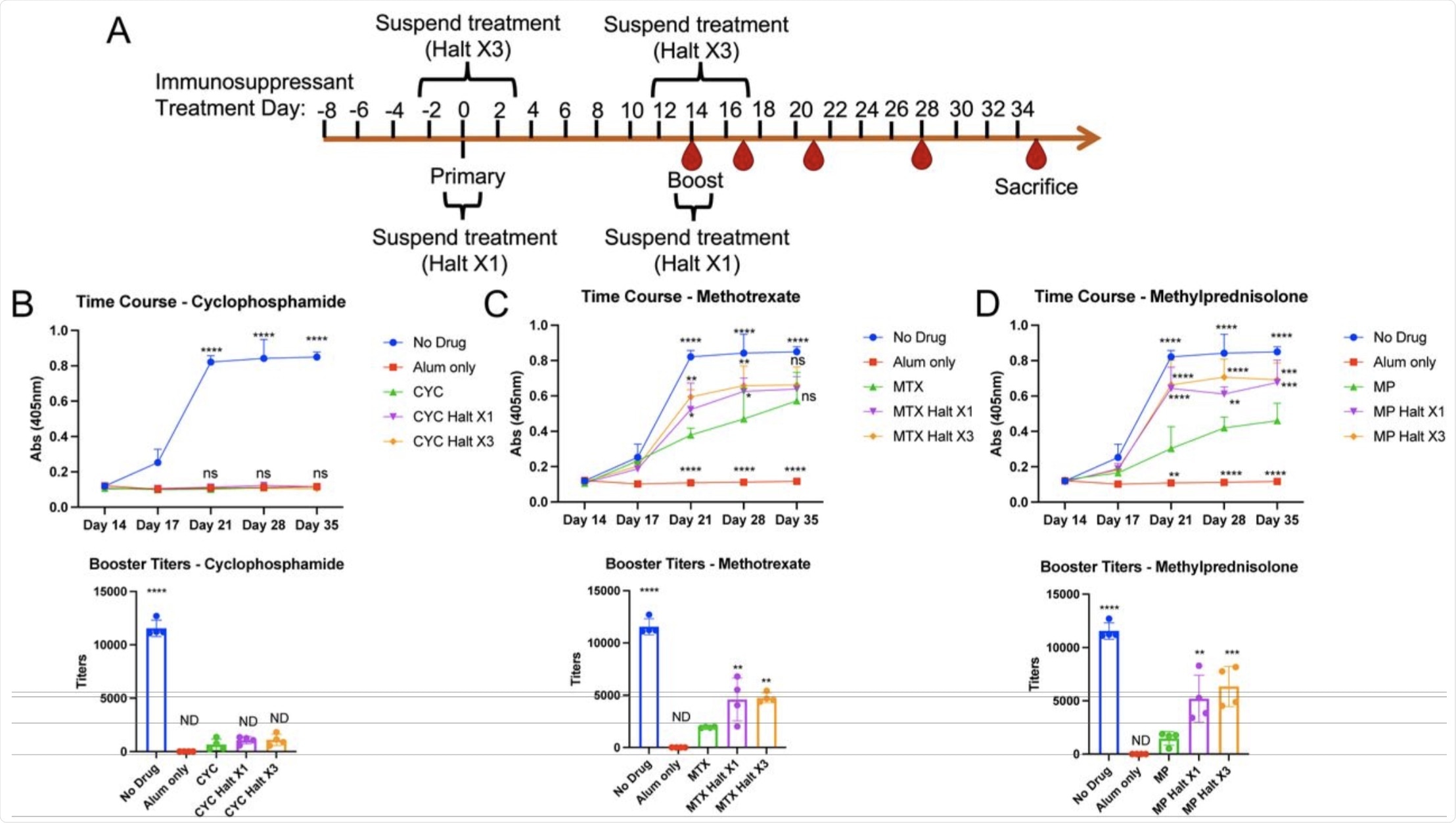 Changes in antibody titers to SARS-CoV-2 spike protein following continuous or temporarily-suspended immunosuppressant administration. A. Schematic of intraperitoneal SARS-CoV-2 spike protein immunization and immunosuppressant administration. Spike protein was injected at 0.5μg/50μl alum per mouse. Immunosuppressants were administered intraperitoneally at the indicated timepoints according to the concentrations in Supplementary Table 2. Within each immunosuppressant group, 4 mice did not receive the respective immunosuppressant on days 0 and 14, and 4 mice did not receive immunosuppressants on days - 2, 0, 2, 12, 14, and 16. B-D. Serum was obtained from immunosuppressant-treated mice at days 14, 17, 21, 28 and 35. ELISA was performed to determine serum IgG against spike protein. Multiple serial serum dilutions were performed, with each dilution’s set of samples (including all timepoints) on one 384-well plate. Input was individual OD values at 405nm absorbance. Shown are ELISA data using 1:3200 serum dilutions from 4 mice/group (top panels) and calculated booster titers (Day 21) based on regression curves from serial dilutions (bottom panels). Statistical values were determined as compared to continuous drug treatment (A. CYC; B. MTX; C. MP).
Changes in antibody titers to SARS-CoV-2 spike protein following continuous or temporarily-suspended immunosuppressant administration. A. Schematic of intraperitoneal SARS-CoV-2 spike protein immunization and immunosuppressant administration. Spike protein was injected at 0.5μg/50μl alum per mouse. Immunosuppressants were administered intraperitoneally at the indicated timepoints according to the concentrations in Supplementary Table 2. Within each immunosuppressant group, 4 mice did not receive the respective immunosuppressant on days 0 and 14, and 4 mice did not receive immunosuppressants on days - 2, 0, 2, 12, 14, and 16. B-D. Serum was obtained from immunosuppressant-treated mice at days 14, 17, 21, 28 and 35. ELISA was performed to determine serum IgG against spike protein. Multiple serial serum dilutions were performed, with each dilution’s set of samples (including all timepoints) on one 384-well plate. Input was individual OD values at 405nm absorbance. Shown are ELISA data using 1:3200 serum dilutions from 4 mice/group (top panels) and calculated booster titers (Day 21) based on regression curves from serial dilutions (bottom panels). Statistical values were determined as compared to continuous drug treatment (A. CYC; B. MTX; C. MP).
Implications
Current guidelines from the American College of Rheumatology suggest withholding certain immunosuppressant treatments before COVID-19 vaccination, which is validated by the findings from this study.
The current COVID-19 pandemic has highlighted the definite need for consistent and progressive research into immunosuppressant therapeutics and vaccinations. The authors have demonstrated through practical study that careful monitoring of immunosuppressant drugs around the time of vaccination can improve the SARS-CoV-2 vaccine response.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Paschall, A. V., Ozdilek, A., Briner, S. L., et al. (2021). Modulation of immunosuppressant drug treatment to improve SARS-CoV-2 vaccine efficacy in mice. biorxiv. doi:10.1101/2021.09.28.462156. https://www.biorxiv.org/content/10.1101/2021.09.28.462156v1.
- Peer reviewed and published scientific report.
Paschall, Amy V., Ahmet Ozdilek, Sydney L. Briner, Melinda A. Brindley, and Fikri Y. Avci. 2022. “Modulation of Immunosuppressant Drug Treatment to Improve SARS-CoV-2 Vaccine Efficacy in Mice.” Vaccine 40 (6): 854–61. https://doi.org/10.1016/j.vaccine.2021.12.058. https://www.sciencedirect.com/science/article/pii/S0264410X21016662.