The burden of infectious diseases in the elderly population is high due to immunosenescence, which is a process of immune dysfunction that often occurs in older people. As a result, this patient population is often at a greater risk of developing infectious diseases, autoimmune diseases, and malignant tumors. Immunosenescence also causes an impaired response to vaccination, as evident in the reduced vaccine efficacy observed following the immunization of patients older than 65 years with the inactivated influenza virus.
The underlying mechanism of immunosenescence includes a reduction in naive T-cells due to thymic involution, the accumulation of memory cells, and inflammaging, which is an increased presence of inflammatory molecules. To improve vaccine efficacy, research studies assess the antigen dose, adjuvant formula, and use of live inactivated vaccination.
Since age is a major risk factor for mortality following infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the coronavirus disease 2019 (COVID-19), older individuals received priority consideration for COVID-19 vaccination in most nations across the world.
Notably, previously published efficacy data of the Pfizer-BioNTech mRNA vaccine against SARS-CoV-2 was based on a study population in which 4% of all participants were over the age of 75 years. The current study looks at the immune response in elderly people, assessing the serological and cellular response against the virus and its variants.
The study
The Pfizer–BioNTech BNT162b2 vaccine is a strongly immunogenic COVID-19 vaccine that consists of a nucleoside-modified mRNA encoding a mutated form of the full-length spike protein of SARS-CoV-2.
The researchers of the current study recruited 100 elderly participants, between the ages of 80 and 96 years who had received the BNT162b2 vaccine with a 3-week interval between their first and second doses. Notably, 58% of the study participants were female.
After receiving the initial and final vaccine doses, the researchers collected blood samples from the participants for the quantitative measurement of spike (S) responses and to determine their nucleocapsid (N)-specific antibody serostatus.
Study findings
Taken together, strong S-specific antibody responses were observed in all study participants, with 98% above 1 in 50. Thus, this response exceeded the response seen following natural infection with SARS-CoV-2.
The N-specific antibody serostatus provided information to the researchers as to whether the study participants had a prior infection with SARS-CoV-2. To this end, 10% of the study cohort was found to have a prior infection with SARS-CoV-2, of which only 4 of the individuals reported symptoms with their previous infection.
Importantly, individuals with a prior SARS-CoV-2 infection had a median S-specific antibody titer that was 28-fold higher than those without prior infection.
After receiving their first vaccination, 63% of the samples exhibited positive antibody responses. Comparatively, after their second vaccination, 96% of the test subjects had detectable antibody responses.
Between the first and second doses among individuals who were previously infected with SARS-CoV-2, their antibody ratio exhibited a modest rise of 6%. Thus, this finding reaffirms previous studies supporting a single vaccine dose in individuals with prior SARS-CoV-2 infection.
S-specific T-cell responses were observed in 63% of participants, whereas the responses against both the S1 and S2 domains were equivalent. In individuals with previous SARS-CoV-2 infection, a 3-fold stronger T-cell response against the S protein after vaccination as compared to those without a history of COVID-19. A similar response was also observed against the N and membrane (M) proteins in this group.
Because there is a strong correlation between the humoral and cellular immunity in a natural infection, the researchers suggested assessing how the suboptimal antibody or cellular responses relate in people who fail to gain complete clinical protection from infection following vaccination.
Natural protection
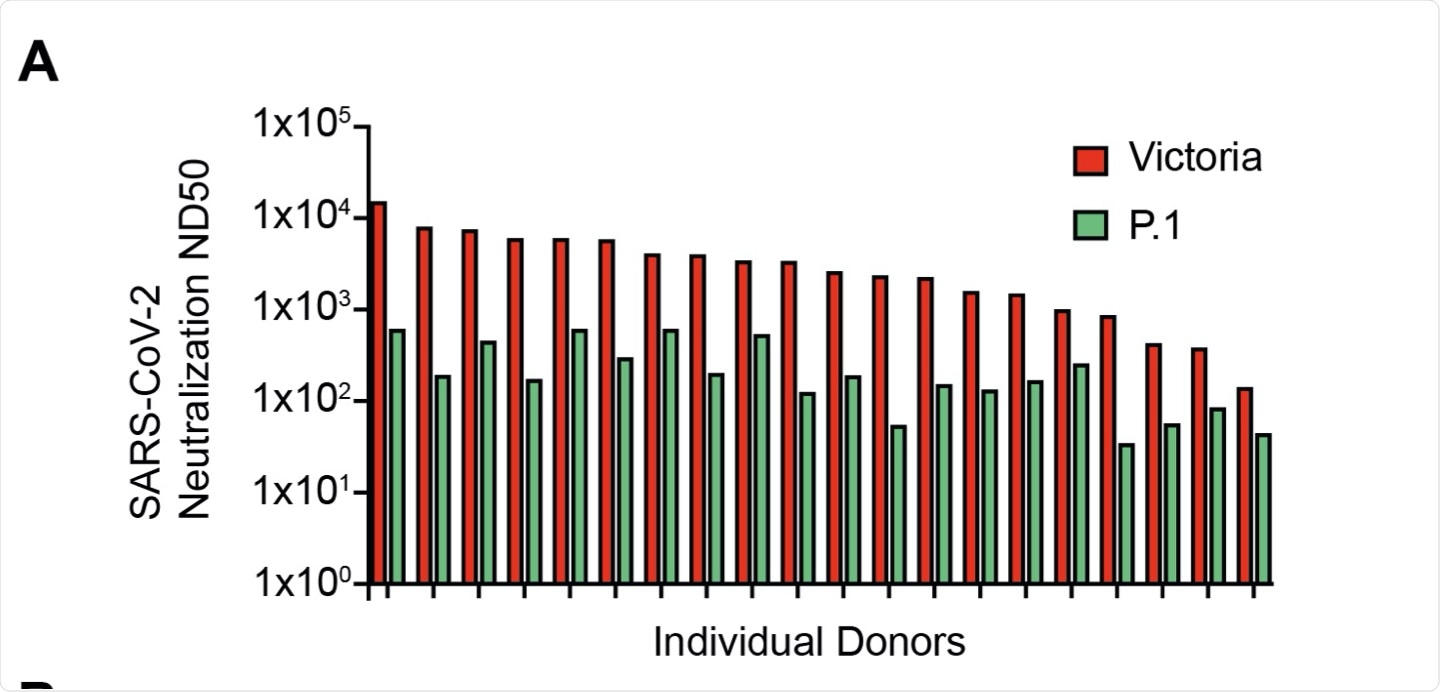
To assess the natural protection post-vaccination, the researchers tested live virus neutralization of the Victoria strain correlated with global spike-specific response. To this end, the researchers found a 14-fold reduction in the neutralization titer against the P.1 VOC and a 3.8-4.8-fold decrease with the live virus.
Based on these observations, the researchers inferred that vaccinated older people are unlikely to be susceptible to the emerging variants.
Limitations
The researchers pointed out a major limitation of the study as the absence of data on pre-vaccinated samples from participants, due to the quick rollout of vaccination programs among the elderly population. It also reflects the challenge of operating within vaccine centers during a national lockdown.
Conclusion
The findings from this study highlight the importance of vaccinating older people, as antibody responses remained robust in donors up to 96 years of age. Thus, this study shows that the mRNA BNT162b2 vaccine efficacy induces a humoral response that is independent of age with the older population.
Journal reference:
- Parry, H., Tut, G., Bruton, R., et al. (2021). mRNA vaccination in people over 80 years of age induces strong humoral immune responses against SARS-CoV-2 with cross neutralization of P.1 Brazilian variant. Microbiology and Infectious Disease. doi:10.7554/eLife.69375.