With the initiation of mass vaccination against COVID-19 worldwide in December 2020, regulatory agencies, academia, pharmaceutical companies, and other stakeholders are working on various safety surveillance strategies based on observational data.
The vACCine covid-19 monitoring readinESS (ACCESS) project in Europe is funded by the European Medicines Agency and includes a protocol to evaluate background rates of conditions representing potential adverse events of special interest (AESI) from observational data.
Studying an age- and sex-stratified Swedish cohort for AESI outcomes through the COVID-19 pandemic
Recently, researchers from Sweden reported population-based, age- and sex-specific background incidence rates of conditions that represent potential COVID-19 vaccine adverse events of special interest (AESI) in the general Swedish population with the help of registered data. This study is published on the medRxiv* preprint server.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The researchers analyzed an age- and sex-stratified, random 10% sample of the Swedish population on 1 Jan 2020, followed up for one year for AESI outcomes through the COVID-19 pandemic, before vaccinations were approved or started.
The following outcomes were selected and defined by the authors based on information from regulatory authorities, previous studies, large-scale adverse events initiatives: febrile seizure, aseptic meningitis, Kawasaki syndrome, arthritis, post-infectious arthritis, myocarditis, myocardial infarction, ARDS, stroke, hemorrhagic stroke, ischemic stroke, venous thromboembolism, kidney failure, pulmonary embolism, liver failure, `erythema multiforme, autoimmune thyroiditis, disseminated intravascular coagulation, and appendicitis.
They determined incidence rates stratified by age, sex, and time period and classified them according to the following Council of International Organizations of Medical Sciences (CIOMS) categories: very common, common, uncommon, rare, or very rare.
Incidence rates of adverse events varied greatly by age and in some cases sex
A total of 972,723 subjects representing the Swedish national population on 1 Jan 2020 were included in the study. They found that the incidence rates of AESI varied significantly by age and, in some cases, sex. Many common AESIs increased, as expected, with age, while some AESIs such as appendicitis, autoimmune thyroiditis, aseptic meningitis, Kawasaki syndrome and MISC were more common among young people. On the other hand, other AESIs such as myocarditis and erythema multiforme showed a flatter age pattern.
As a result, the CIOMS classification for AESIs widely varied based on age. The findings showed considerable variability across the 4 quarters of 2020 for some AESI rates, which may be potentially related to the pandemic waves, healthcare system overload, other healthcare delivery effects, or seasonal variation. In conclusion, age, sex, and timing of rates are important variables to consider while comparing background AESI rates to corresponding rates observed with COVID-19 vaccines.
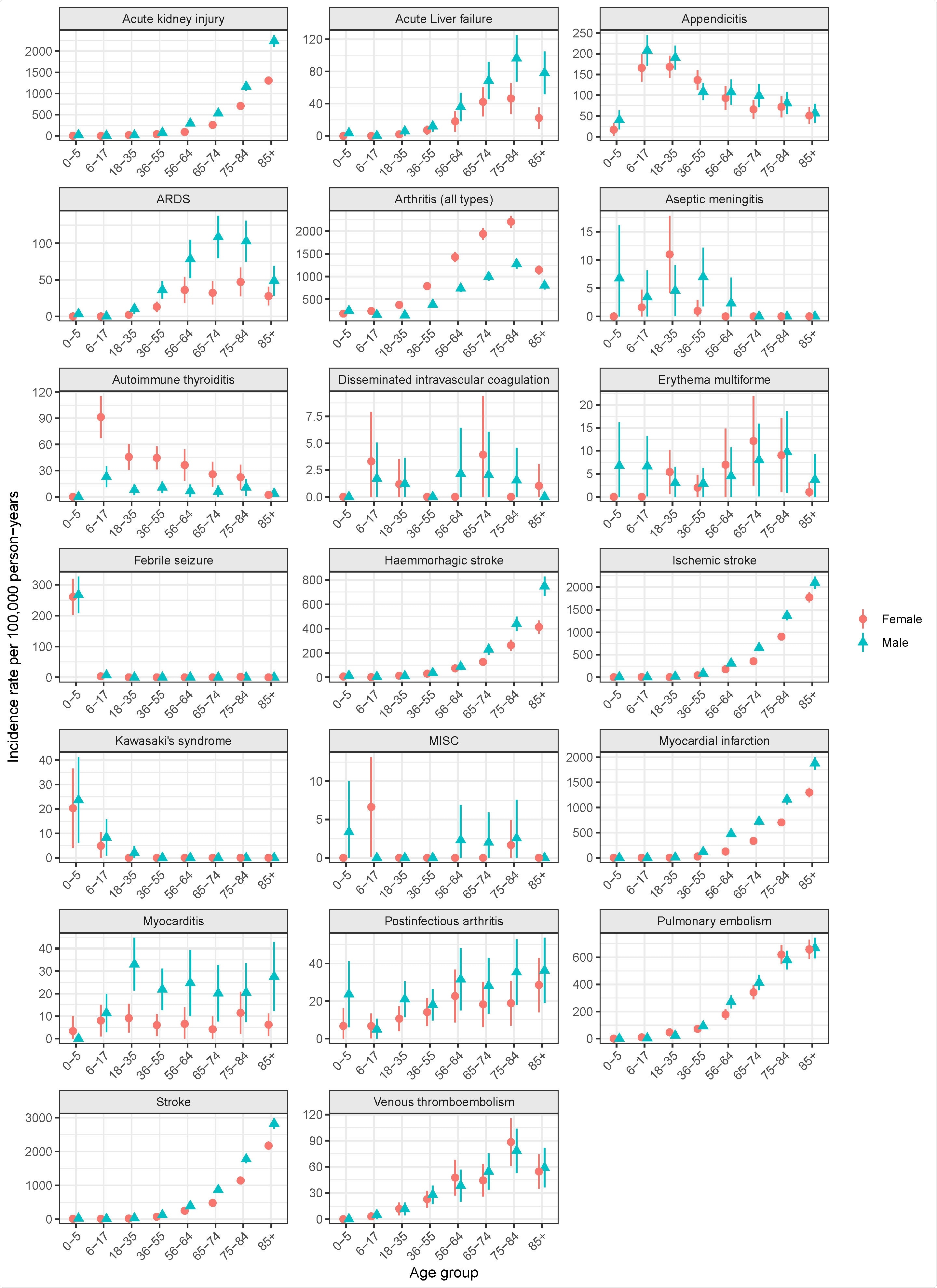
Age- and sex-stratified incidence rates of 14 adverse events of special interest (AESIs) per 100,000 person-years (with 95% confidence intervals) in the Swedish population
Demographic comparability and population heterogeneity must be taken into account while evaluating AESIs
This study reported descriptive epidemiology for many potential AESIs for safety follow-up of COVID-19 vaccines from a random Swedish population. The work described baseline rates of the AESIs during 2020 overall and by quarter and the results show some crucial points for future vaccine safety studies.
“The clear trends over quarters seen for some AESI during the pandemic in Sweden, with variable patterns, imply that comparing rates across appropriate time periods will be crucial.”
According to the authors, the considerable variability of rates by age and for some outcomes also by sex, indicate the importance of sufficient demographic comparability for vaccinated and non-vaccinated groups compared. It is also essential to ensure adequate adjustment or standardization for age and sex while using background rates for safety surveillance.
Recent large international studies have shown significant heterogeneity across populations; for instance, data from the ACCESS project showed similar magnitudes of heterogeneity in background rates. The OHDSI group study reported variability across databases and sites. This emphasizes the fact that population heterogeneity must be taken into account while evaluating AESIs.
“These background rates provide useful real-world clinical context for activities aiming at vaccine monitoring and ensuring patient safety as Covid-19 vaccines are applied to combat the pandemic worldwide.”

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Adverse events of special interest for COVID-19 vaccines - background incidences vary by sex, age and time period and are affected by the pandemic Fredrik Nyberg, Magnus Lindh, Lowie EGW Vanfleteren, Niklas Hammar, Björn Wettermark, Johan Sundström, Ailiana Santosa, Brian K Kirui, Magnus Gisslén, medRxiv, 2021.10.04.21263507; doi: https://doi.org/10.1101/2021.10.04.21263507, https://www.medrxiv.org/content/10.1101/2021.10.04.21263507v1