The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is not the same virus that was first encountered in Wuhan, China. As the world adapted to the presence of SARS-CoV-2 with face masks and social distancing, the virus adapted to counter these measures.
In a new study published in Virus Genes, German researchers discovered that mutations accumulate, especially in immunocompromised patients. Heavily mutated variants, such as Beta and others emerging during plasma therapy, show decreased sensitivity or complete insensitivity towards neutralization.
 Study: Sensitivity of two SARS-CoV-2 variants with spike protein mutations to neutralising antibodies. Image Credit: PHOTOCREO Michal Bednarek / Shutterstock.com
Study: Sensitivity of two SARS-CoV-2 variants with spike protein mutations to neutralising antibodies. Image Credit: PHOTOCREO Michal Bednarek / Shutterstock.com
The findings of the current study indicate that monoclonal antibodies and current coronavirus disease 2019 (COVID-19) vaccines, both of which are based on the wild-type spike protein, may not be as effective in patients infected with a SARS-CoV-2 variant or with prolonged infection.
Researchers suggest adapting vaccines and other COVID-19 treatments to deal with SARS-CoV-2 variants will require future surveillance of emerging and circulating variants.
About the study
The current study evaluated neutralizing sensitivity of spike protein mutations in two SARS-CoV-2 variants. Samples were collected from patients with COVID-19 infection from April to May 2020 undergoing convalescent plasma therapy.
Whole-genome sequencing helped to pinpoint the isolated viral samples to the B.1.351 lineage. MUC-IMB-B.1.351 was one variant collected from a patient with acute COVID-19 infection. Another variant, MUC-484, was isolated from an immunocompromised patient with prolonged COVID-19 infection.
SARS-CoV-2 mutations differ from the original Wuhan strain
In the MUC-IMB-B.1.351 variant, 26 mutations were found on the spike protein. Ten of the 26 spike protein mutations were not present in the original Wuhan SARS-CoV-2 genome.
Notable spike protein mutations included K417N, E484K, N501Y, and D614G. In addition, there were also amino acid changes in D80A and D215G in the N-terminal domain of the spike protein.
In the MUC-484 variant, there were three mutations found on the spike protein. These included D614G, E484K, and an in-frame deletion. The K417N, E484K, and N501Y mutations have been associated with other variants of concern for their notorious ability to escape antibody responses.
Some, but not all, mutations were also found in the SARS-CoV-2 Beta variant, thereby suggesting a transitional variant.
MUC-IMB-B.1.351 variant exhibit decreased neutralizing sensitivity
Taken together, 73 of the 89 convalescent plasma samples containing neutralizing titers effectively neutralized the original SARS-CoV-2 strain. However, a majority of samples could not neutralize MUC-IMB-B.1.351 with a 99% mean loss of neutralizing activity. Nine samples that did retain neutralizing power showed a significant 91.5% median reduction in neutralizing activity.
The researchers suggest MUC-IMB-B.1.351 is highly insensitive to neutralization because of a buildup of substitutions, including K417N and N501Y. Additionally, an accumulation of mutations may induce a synergistic effect, thus enhancing their effects on each other.
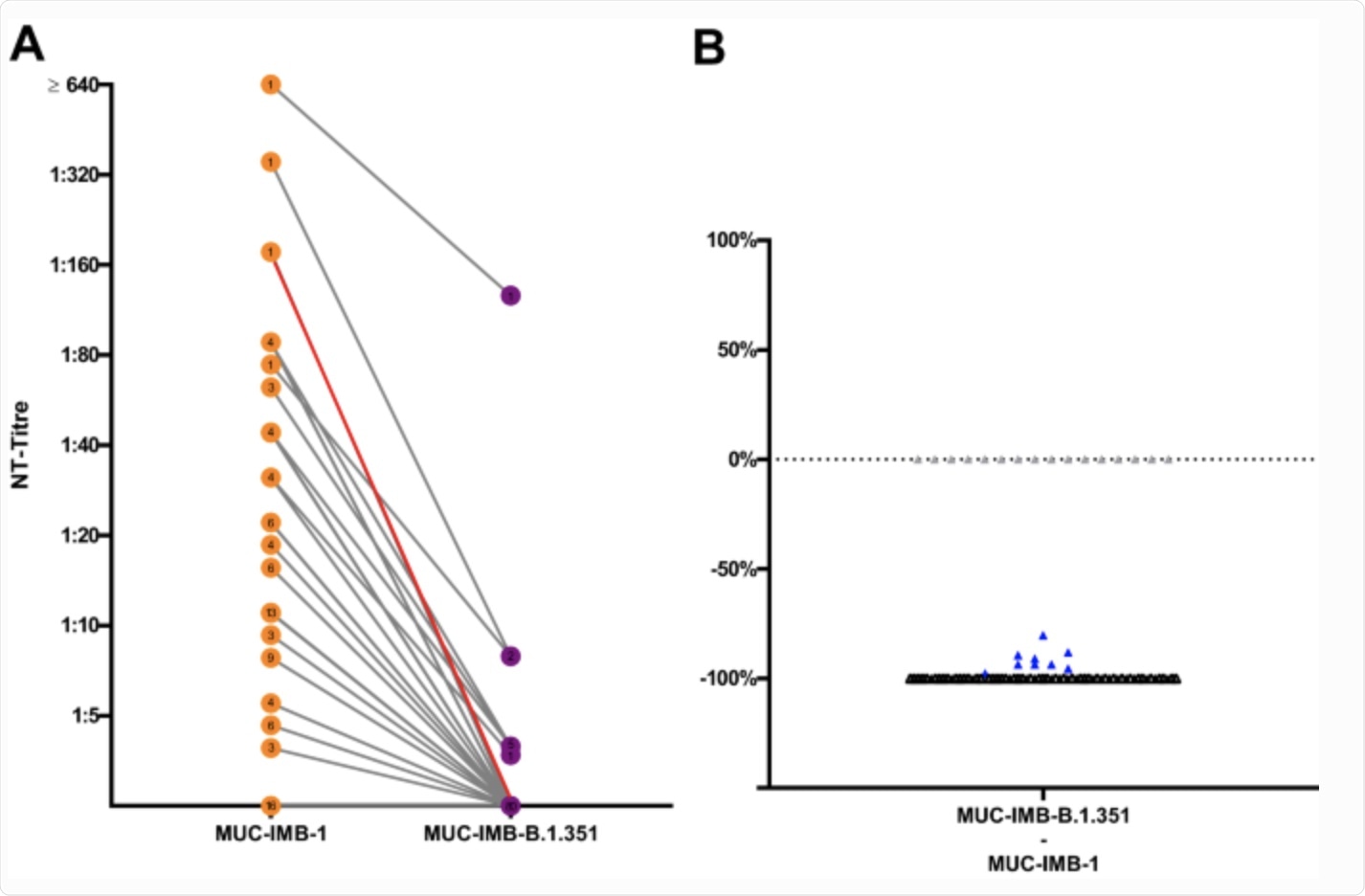 Comparison of MUC-IMB-1 and MUC-IMB-B.1.351 demonstrates a significant decrease in sensitivity towards NAbs (A) Side by-side comparison of NAbs titres against WT (orange) and MUC-IMB-B.1.351 (purple). The greatest decrease was observed in a sample, which dropped from an initial titre of 177 to no neutralising effect against B.1.351 (red). (B) All but nine samples (blue) show a complete loss of neutralising activity (black). All samples that tested negative for NAbs against WT remained negative when tested against MUC-IMB-B.1.351 (grey). (∑ samples: 89, the number of overlapping samples is shown in the respective circle).
Comparison of MUC-IMB-1 and MUC-IMB-B.1.351 demonstrates a significant decrease in sensitivity towards NAbs (A) Side by-side comparison of NAbs titres against WT (orange) and MUC-IMB-B.1.351 (purple). The greatest decrease was observed in a sample, which dropped from an initial titre of 177 to no neutralising effect against B.1.351 (red). (B) All but nine samples (blue) show a complete loss of neutralising activity (black). All samples that tested negative for NAbs against WT remained negative when tested against MUC-IMB-B.1.351 (grey). (∑ samples: 89, the number of overlapping samples is shown in the respective circle).
MUC-484 variant reduced neutralizing activity
Neutralizing efficacy dropped in most plasma samples against the original SARS-CoV-2 strain and the MUC-484 variant. About 44 samples were able to neutralize the original SARS-CoV-2 strain but showed no neutralizing activity against MUC-484.
A total of 64 samples were successful in neutralizing MUC-IMB-1. However, of the 64, only 20 could neutralize MUC-484. In these samples, 18 showed a median 89.6% decrease in neutralizing antibody titers.
The researchers observed increased neutralization with two samples against MUC-484. They theorize this may be due to rare cases of polyvalent antibody formation that could increase neutralization against variants.
MUC-484 had the E484K mutation and resulted in an overall median loss of 90.6% neutralizing efficacy as compared to the 99% loss of neutralizing efficacy in MUC-IMB-B.1.351.
“These findings underline that the E484K mutation alone is vigorously reducing neutralization sensitivity due to its enhancement of hACE2 binding whilst reducing the binding affinities of neutralizing monoclonal antibodies.”
The researchers also suggest that having accumulated immune-escape mutations or a single one in the spike protein is enough to reduce neutralizing responses against SARS-CoV-2.
Study limitations
The current study measured neutralizing titers of donated samples; however, the number of samples themselves is low. The low number of tested samples may overestimate the variants’ immune escape potential.
Another study limitation was the possibility of a preexisting cellular immune response. There may have been more cross-reactivity in the cellular immune response than neutralizing antibodies.