My name is Aryn Gittis. I started studying Parkinson’s disease as a postdoc in 2008 and it has been the central focus of my lab since starting at Carnegie Mellon University in 2012.
Statistics show that more than 10 million people worldwide are living with Parkinson’s disease, yet there is still no cure. Why is this and what treatments are currently available to help relieve the symptoms of Parkinson’s disease?
Parkinson’s disease is a complex disorder. It originates with the degeneration of dopamine neurons, which then changes how brain cells operate throughout the brain. This makes it hard to figure how out how to intervene in a way that restores movement without causing side effects. Because patients often do not develop the motor symptoms used for diagnosis until almost 70% of their dopamine has been lost, treatment begins late in the disease process after a number of brain systems are already affected.
As a result, there isn’t one single treatment that can reverse all PD symptoms. Even treatments like levodopa, which try to restore dopamine levels, produce side effects over time because the brain is constantly adapting to changes in its chemical environment.
Image Credit: Astrid Gast/Shutterstock.com
What is deep brain stimulation (DBS) and why do researchers still not fully understand how it works?
Deep brain stimulation is a technique in which an electrode is implanted into the brain and stimulation is used to disrupt abnormal brain rhythms associated with a disease.
What are some of the current limitations to DBS and its application to Parkinson’s?
Currently, DBS only works to alleviate symptoms when stimulation is on. As soon as stimulation is turned off, symptoms return. So the treatment is masking the symptoms, but not fixing the underlying problems.
Currently, in order for patients to get relief from their symptoms, they need to receive continuous simulation but your new research may be able to change that. How have you made this possible?
We found a way to deliver stimulation in a manner that is much more specific than what has been used previously. With our approach, 30 minutes of stimulation was enough to restore movement, and then the stimulator could be left off for hours without symptoms returning.
Can you describe how you carried out your latest research into DBS and Parkinson’s disease? What did you discover?
My lab studies how brain cells communicate during movement and why the loss of dopamine, as occurs in Parkinson’s disease, disrupts their communication. Through this basic research, we observed how different types of neurons responded to electrical stimulation and found a brief window, right after stimulation was turned on, where some neurons sped up and some slowed down. This was the same pattern of neural activity that had produced long-lasting therapeutic effects in a previous study, but that research was done using a technique called optogenetics, which is not possible to use in humans.
But if we left stimulation on for more than a few seconds, all the neurons started to speed up and the specificity was lost. This suggested to us that if we patterned our electrical stimulation correctly – using brief bursts of stimulation, rather than continuous stimulation as used during conventional DBS, we could use DBS to drive a specific pattern of neural activity that can induce long-lasting therapeutic responses.
When we tested this idea in Parkinsonian mice, we found that burst-DBS increased movement to the same degree as conventional DBS protocols, but allowed mice to keep moving after stimulation was turned off, whereas mice who received the conventional DBS protocol stopped moving as soon as stimulation was turned off.
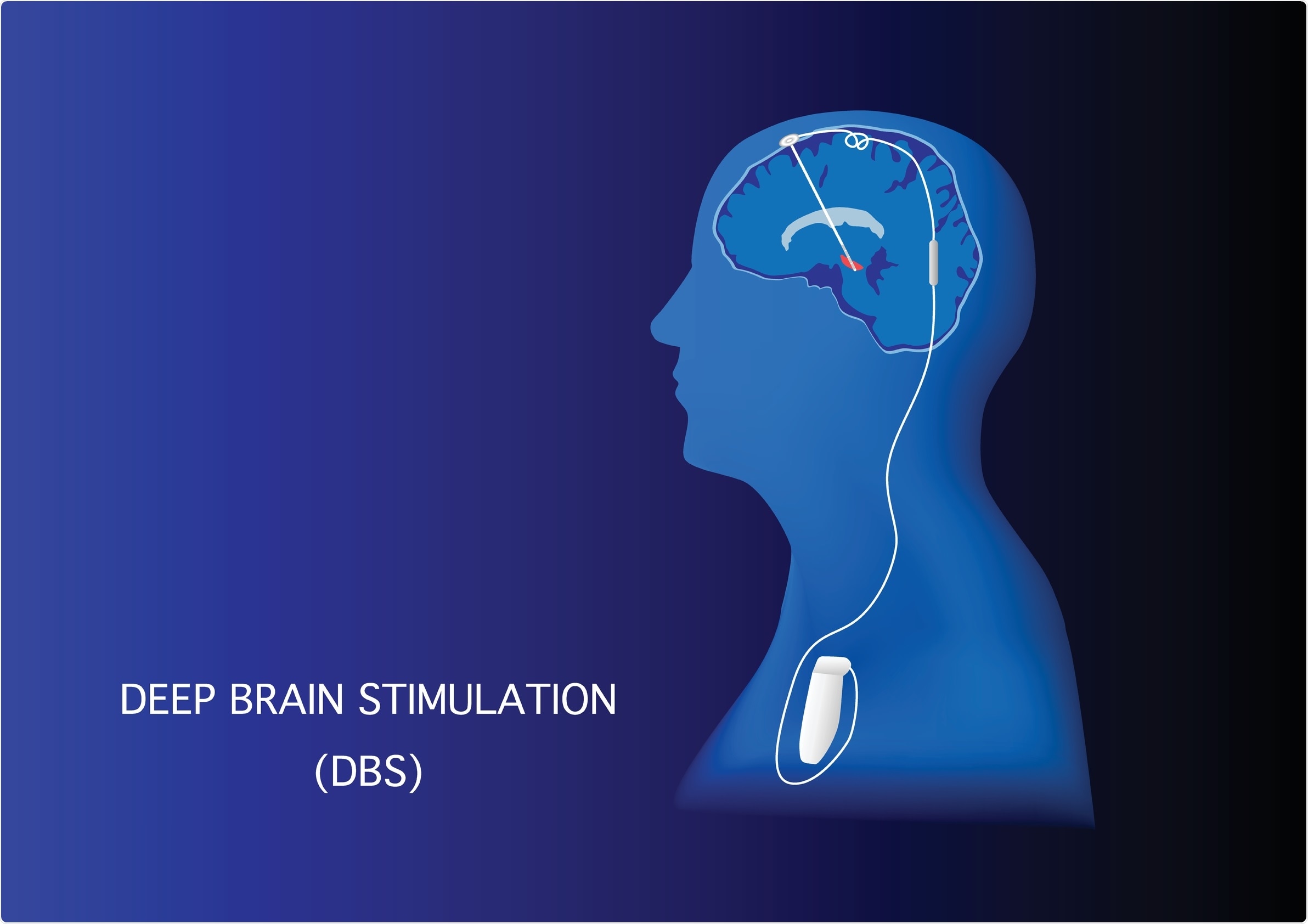
Image Credit: Teeradej/Shutterstock.com
In a world where much scientific and medical attention has been paid to the ongoing COVID-19 pandemic, why is it still just as important to continue to raise awareness for, and research, the causes and treatment options for Parkinson’s?
Parkinson’s disease is the second most common neurological disorder after Alzheimer’s disease and affects nearly 1 million people in the U.S. As the U.S. population ages, this number is expected to increase. The rapid development of vaccines against COVD-19 was made possible through decades of basic research which was then translated with unprecedented speed by partnerships aligned across academic labs, industry, and government.
Rather than diverting attention away from the study of other diseases, the world’s response to COVID should serve as an inspiration for what can be accomplished when we go all-in on treating a disease. COVID-19 will probably remain in circulation for years to come, but its threat to public health will diminish.
Conversely, public health burdens from neurological and neuropsychiatric diseases are continuing to grow. But the basic research is there, and if we focus on translating this treatment from the research lab to the clinic, I think we’ll see major breakthroughs in how we treat these diseases over the next 10 years.
How will your research help to significantly advance research into Parkinson’s disease?
We have found a stimulation pattern that can already be programmed using existing devices that can reduce stimulation time and provide longer-lasting therapeutic effects. We don’t yet fully understand why the effects of burst stimulation are so long-lasting, but one exciting possibility is that it is treating underlying circuit dysfunction, rather than simply masking symptoms. This is the research that we are doing now.
We want to study the short-term and long-term effects of burst DBS throughout the motor system. Our hope is that we can use this stimulation to retrain the motor system to function despite the continued absence of dopamine.
Where can readers find more information?
- My lab website: https://labs.bio.cmu.edu/gittis/
- The Neuroscience Institute, CMU: https://www.cmu.edu/ni/
- Mellon College of Science, CMU: https://www.cmu.edu/mcs/
About Dr. Aryn Gittis
Aryn Gittis, Ph.D., is an Associate Professor in the Department of Biological Sciences and the Neuroscience Institute at CMU. She received her undergraduate degree from Brandeis University in 2001 and was a Fulbright Scholar in France from 2001-2002. She received her Ph.D. from UCSD in 2008 where she studied with Sascha du Lac, and then completed a postdoctoral fellowship in Anatol Kreitzer’s lab at the Gladstone Institute/UCSF in 2012. For her postdoctoral work, she was awarded a K99 from NINDS and was a 2012 Finalist for the Eppendorf & Science Prize for Neurobiology..jpg)
She joined CMU in 2012, where her lab uses electrophysiology, optogenetics, and computational approaches to study the progression of neural circuit dysfunction in mouse models of Parkinson’s disease, with the goal of developing strategies to guide therapeutic plasticity that can repair circuit dysfunction and restore movement.
She has received numerous awards for this work, including a NARSAD Young Investigator Grant in 2013, the Janett Rosenberg Trubatch Career Development Award from the Society for Neuroscience in 2018, and was a Finalist for the Science and PINS Prize for Neuromodulation in 2018.