Earlier reports have suggested the high effectiveness of messenger ribonucleic acid (mRNA) coronavirus disease (COVID-19) vaccines at preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, their efficacy against mild COVID-19 disease appears to wane over time. Thus, the need for additional/booster vaccine doses is being contemplated.
An additional vaccine dose may particularly benefit individuals at high risk of breakthrough infections or severe COVID-19, like immunocompromised patients. Nevertheless, it is necessary to monitor the safety of additional vaccine doses.
Extensive research has shown that the standard two-dose regimen of mRNA-based COVID19 vaccines is safe. Data from smaller trials depict that additional vaccination doses might also be safe.
About the study
A new study published on the medRxiv* preprint server aimed to determine the safety of additional vaccine doses among individuals who previously received the standard two-dose regimen of mRNA-based COVID-19 vaccines.
This retrospective study included adults within the Mayo Clinic Enterprise who were vaccinated with three doses of United States Food and Drug Administration (FDA)-approved mRNA-based COVID-19 vaccines from December 1, 2020, to October 17, 2021. Participants received the first two doses of BNT162b2 or mRNA-1273 according to the emergency use authorization (EUA) protocol.
The study participants were administered a third dose of the same vaccine type as the original two doses at least 28 days after the second dose. All subjects were followed up for at least 14 days after their third vaccine dose.
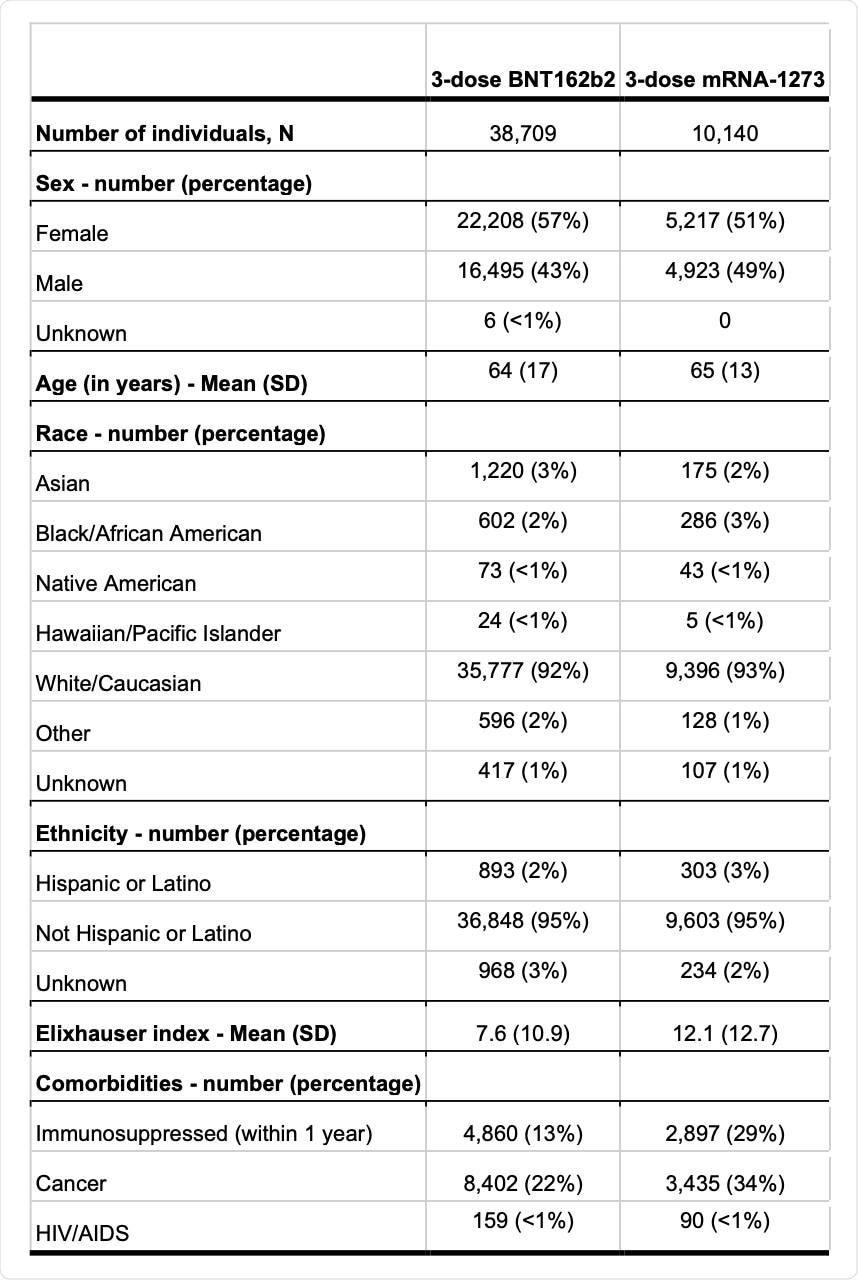 Demographic and tracked comorbidities of the study participants.
Demographic and tracked comorbidities of the study participants.
Study findings
No significant difference was observed in the reporting of severe adverse events; however, there was a significant increase in reporting for most low-severity adverse events after the patients received the third COVID-19 vaccine dose as compared to the previous doses.
The most common adverse events reported after the third vaccine dose were fatigue (4.92%), lymphadenopathy (2.89%), nausea (2.62%), headache (2.47%), arthralgia (2.12%), myalgia (1.99%), diarrhea (1.70%), erythema (1%), fever (1.11%), vomiting (1.10%), chills (0.47%), and soreness (0.36%).
Upon utilizing the risk difference (RD), it was found that aS compared to the second dose, there was increased reporting of the most common adverse events for both BNT162b2 and mRNA-1273 vaccines after the third dose. Yet, no vaccine-specific adverse events were recorded.
Comparatively, reporting of severe adverse events was rare after the third dose and was not significantly higher than the frequency of events reported after the second dose. After the third vaccine dose, four patients reported pericarditis, two patients reported anaphylaxis, and one patient reported myocarditis. Moreover, the significant risk for these adverse events did not appear to have increased after the third dose than that after the second dose.
Upon assessing the rate of emergency department visits, there was only a slight increase within two days after the third BNT162b2 dose, which amounted to 0.29% of vaccine recipients, when compared to after the second dose that reported a rate of 0.2%. Meanwhile, no significant difference in emergency department visits was recorded among mRNA-1273 vaccine recipients.
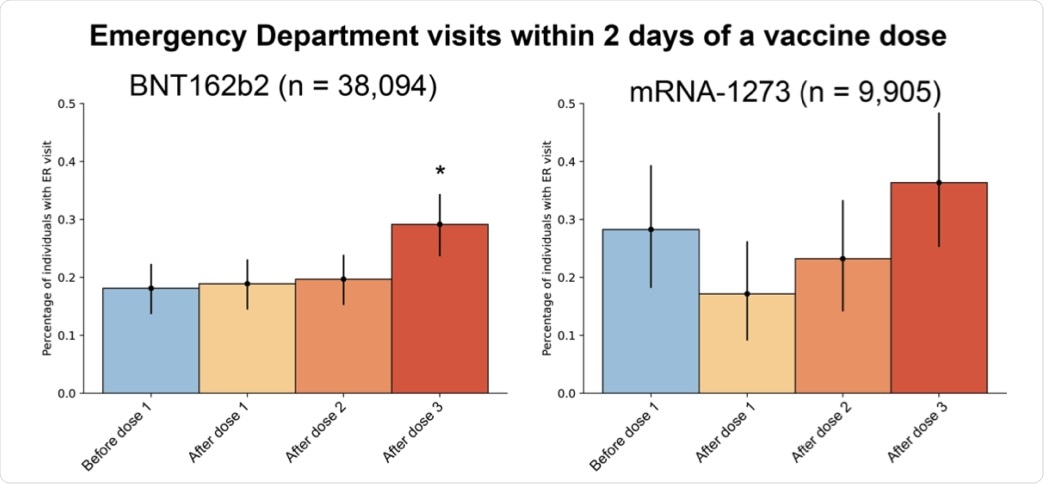 Emergency department visits within 2 days of each vaccine dose. The percentage of 3-dose mRNA vaccine recipients that visited the emergency department within 2 days after the 1st dose (yellow), 2nd dose (orange), or 3rd dose (red), compared with a 2-day period, starting 14-days before their 1st dose (blue). Error bars indicate 95% confidence intervals, and asterisks indicate a significant (two-tailed p-value < 0.05) difference in emergency department visits after vaccination compared with before.
Emergency department visits within 2 days of each vaccine dose. The percentage of 3-dose mRNA vaccine recipients that visited the emergency department within 2 days after the 1st dose (yellow), 2nd dose (orange), or 3rd dose (red), compared with a 2-day period, starting 14-days before their 1st dose (blue). Error bars indicate 95% confidence intervals, and asterisks indicate a significant (two-tailed p-value < 0.05) difference in emergency department visits after vaccination compared with before.
The findings of the current study suggest that a third dose of the same type of vaccine, after either a BNT162b2 or mRNA-1273 primary series, is safe. An increase in the early post-vaccination adverse events was recorded after the third dose as compared to earlier doses. However, these were symptoms of low concern including fatigue, lymphadenopathy, nausea, and diarrhea.
There was no significant increase in electronic health records (EHR) reporting of severe adverse events after a third vaccine dose as compared to after the second dose with an incidence rate compared with previous literature.
Conclusion
The increase in minor adverse events could be due to a stronger immune response elicited by the third vaccine dose as compared to the second and first doses.
The current study provides further evidence that the third dose of COVID-19 vaccination with the same mRNA vaccine, as used in the primary series, is safe in high-risk populations. This study confirms previous findings that propose booster dose safety and effectiveness, while also supporting the implementation of the third dose of mRNA COVID-19 vaccination for at-risk populations.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Niesen, M., Pawlowski, C., O'Horo, J., et al. (2021). Three doses of COVID-19 mRNA vaccination are safe based on adverse events reported in electronic health records. medRxiv. doi:10.1101/2021.11.05.21265961. https://www.medrxiv.org/content/10.1101/2021.11.05.21265961v1
- Peer reviewed and published scientific report.
Niesen, Michiel J. M., Colin Pawlowski, John C. O’Horo, Doug W. Challener, Eli Silvert, Greg Donadio, Patrick J. Lenehan, et al. 2022. “Surveillance of Safety of 3 Doses of COVID-19 MRNA Vaccination Using Electronic Health Records.” JAMA Network Open 5 (4): e227038. https://doi.org/10.1001/jamanetworkopen.2022.7038. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2791034.