The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China, in December 2019. Studies have shown that between 3% to 8% of coronavirus disease 2019 (COVID-19) patients have pre-existing chronic liver disease (LD). Since the beginning of the pandemic, it has been noted that the higher rate of mortality in COVID-19 patients may also be due to chronic liver disease, as it can affect the kinetics of various drugs.
The Child-Pugh (CP) classification system is used to classify the severity of LD based on biological and physiological parameters. Severe chronic LD may alter the hepatic vasculature and cause portal hypertension, potentially leading to portocaval shunting. Shunting decreases first-pass metabolism, which may lead to an increase in the bioavailability of a drug.
Dexamethasone (DEX), a corticosteroid that has been widely used in the treatment of COVID-19 patients, is metabolized by the cytochrome P450 (CYP) enzymatic system, especially by the CYP3A4 isoform. In a previous study, lesser DEX clearance was noted in patients with liver disease as compared to healthy individuals.
To assess enzyme-based drug-drug interactions, a simulation approach is known as physiologically-based pharmacokinetic (PBPK) modeling is being used, which can explain changes in absorption, metabolism, distribution, and elimination (ADME). Importantly, this model considers the body mass index (BMI), body surface area (BSA), height, and weight of individuals in order to allometrically calculate organ and tissue volumes. This information can then be used to calculate blood flows that assist in ADME predictions.
A recent study published on the medRxiv* preprint server used PBPK modeling to demonstrate the use of DEX PKs for the treatment of COVID-19 in patients with liver impairment.
Effect of liver disease on the pharmacokinetics of dexamethasone in COVID-19 patients
The current study based on PBPK modeling focused on the impact of liver disease on the pharmacokinetics of DEX in the treatment of COVID-19. In this study, 100 individuals between the ages of 18-60 years were simulated by the model, and various parameters related to physiological changes like liver size, hepatic blood flow, CP450 expression, plasma protein concentration, and portal vein shunt were integrated into the LD model. All changes were implemented using the Child-Pugh (CP) classification system.
During simulations, it was assumed that the distribution of the drug was immediate and uniform across each compartment, the drug was not reabsorbed from the colon, and that blood flow limited the drug distribution. The oral (PO) and intravenous (IV) forms of DEX were qualified using the clinical data in healthy adults.
Likewise, propranolol (PRO) and midazolam (MDZ) were qualified using PO and IV clinical data in healthy and patients with LD. Subsequently, the qualified model was used to simulate a DEX dose of 6 milligrams (mg) PO and 20 mg IV in patients with various degrees of LD, with and without shunting.
Study findings
The PBPK model was successfully validated in both healthy and LD individuals for all drugs (MDZ, PRO, and DEX). The researchers noted that in patients with LD, the simulated systemic clearance of DEX decreased between 35-60%, while the plasma DEX concentrations increased between 170-400%. Additionally, the AUC ratio between healthy/LD individuals remained comparable at high and low doses of DEX.
The PBPK model was used to predict the exposure of DEX in different phases of LD, which facilitated the prediction of PK in difficult clinical situations related to COVID-19. The model simulations suggested that it is not essential to adjust the dose of DEX in LD patients given the low DEX dose used in the COVID-19 protocol.
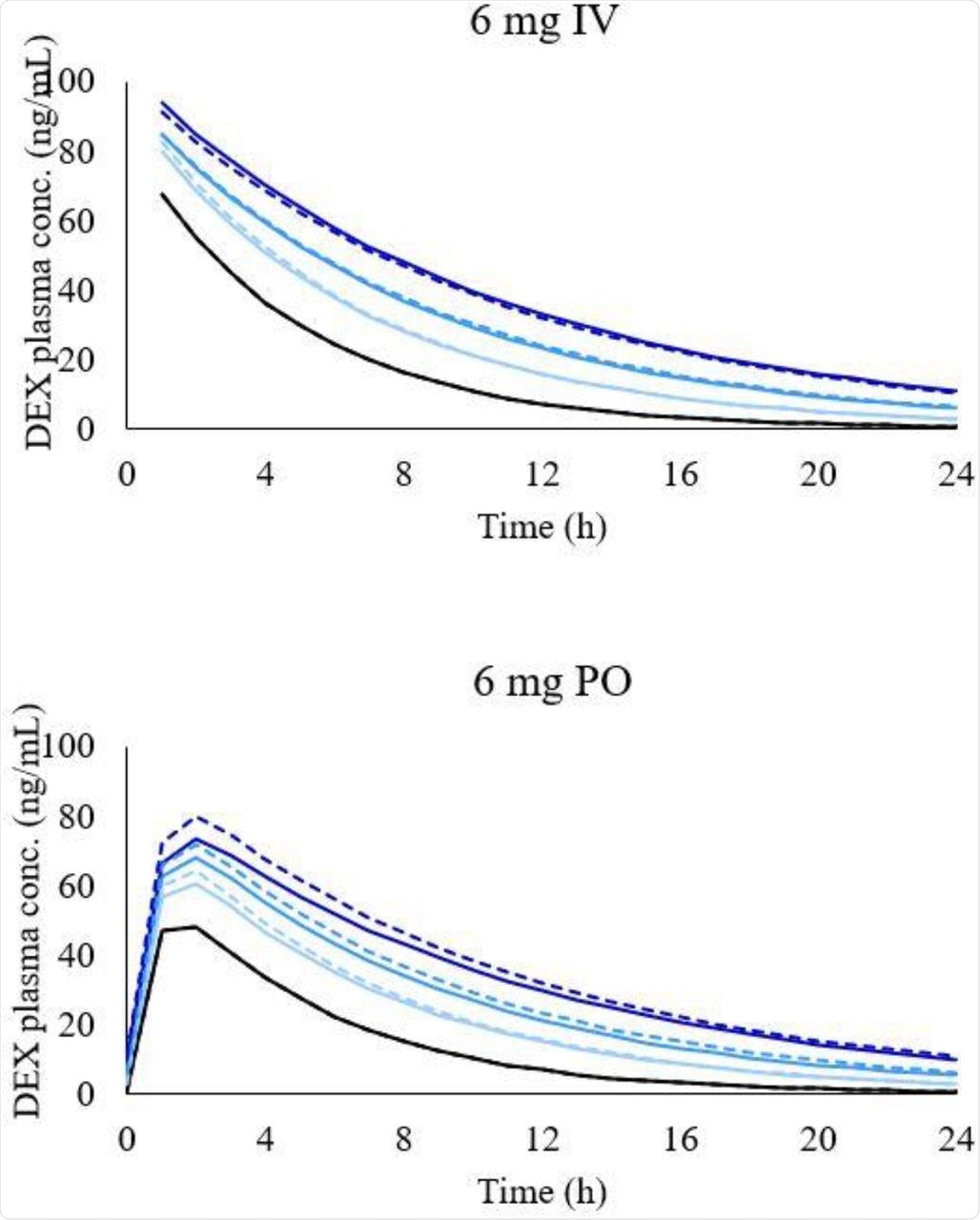 Simulated concentration-time profile of DEX in different LD conditions after 6 mg intravenous administration (graph on the top) and 6 mg oral administration (graph on the bottom). Black line, healthy individuals. Light blue line, CP-A condition, blue line, CP-B condition, and dark-blue line, CP-C condition. Dashed lines represent simulations with shunting and solid lines with no shunting.
Simulated concentration-time profile of DEX in different LD conditions after 6 mg intravenous administration (graph on the top) and 6 mg oral administration (graph on the bottom). Black line, healthy individuals. Light blue line, CP-A condition, blue line, CP-B condition, and dark-blue line, CP-C condition. Dashed lines represent simulations with shunting and solid lines with no shunting.
Limitations
Due to the limitation of data, the current study did not consider the fact that COVID-19 is associated with high cytokine and C-reactive protein levels, which affect the PK of the drug through the downregulation of CYP isoenzymes. Further, the drug-drug interaction between DEX and tocilizumab was not considered, although both drugs have been coadministered during COVID-19 treatment.
Inferences
The researchers studied the increased exposure of DEX across varying phases of LD with the help of PBPK modeling. The results of the study showed that there is no need to adjust the dose in patients with LD, considering a small treatment period, a low dose of DEX used in the COVID-19 protocol, and low hepatic clearance of DEX, although 3 times higher DEX exposure was observed in CP-C individuals.
The outcomes of this study facilitate the management of difficult COVID-19-related clinical scenarios and provide a rational pathway for future PBPK modeling applications in LD patients.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information