Hand washing, mask usage and social distancing were shown to be somewhat effective measures in our fight against the COVID-19 pandemic. However, considering that SARS-CoV-2 dissemination shows a strong association with the mouth, antiseptic mouthwashes have been suggested as an extra preventive step against the disease.
For example, phthalocyanines have shown good inactivation properties against different microbial pathogens, and their potential in various biological and medical applications has been recognized. Accordingly, some studies have already hinted that promising activity of anionic phthalocyanine derivative in a mouthwash protocol against SARS-CoV-2.
A recent study first-authored by Dr. Paulo Sérgio da Silva Santos from the Bauru School of Dentistry of the University of São Paulo went one step further, as it aimed to appraise the antiviral activity and cytotoxicity of anionic phthalocyanine derivative in laboratory conditions, but also to clinically assess its use in hospitalized patients with COVID-19.
Laboratory and clinical evaluation of the mouthwash
In this two-arm study, researchers have evaluated antiviral activity and cytotoxicity of anionic phthalocyanine derivative in vitro. Furthermore, a randomized controlled trial was conducted with a total of 41 hospitalized patients positive for COVID-19.
All patients included in the study have received standard care hospital treatment (non-intensive care) as recommended by the World Health Organization (WHO), together with either an active mouthwash (experimental group of 20 patients) or non-active mouthwash (control group of 21 patients).
This mouthwash intervention that has been used in both patient groups comprised one minute of gargling and rinsing – five times per day until they were discharged from the hospital. Groups were then compared in respect to their age, symptom burden and duration before admission, number of present comorbidities, and the length of hospital stay.
Consequently, the associations between group and age range, sex, comorbidity burden, admission to the intensive care unit and death have also been evaluated in detail. The study was considered triple-blind, as the patients, the examiner and the statistician were all blinded to the treatment groups.
Less symptoms and shorter hospital stay
Laboratory evaluation has revealed that anionic phthalocyanine derivative compound was extremely effective in reducing SARS-CoV-2 viral load in the 1.0 mg/mL (99.96%) to 0.125 mg/mL (92.65%) range – without causing cytotoxicity. In other words, its antiviral efficacy in vitro has been confirmed.
Regarding the clinical trial, the median length of stay for the active mouthwash group was four days, which was significantly shorter when compared to the non-active group (four vs. seven days). In addition, gargling and rinsing with the mouthwash was somewhat helpful in reducing the severity of symptoms and the need for intensive care.
It is also important to emphasize that, according to the safety outcomes, no side effects have been reported by the patients from both study groups. And even though COVID-19 distribution patterns may vary country-wise in different hospitals, there have been no significant changes when comparing experimental and control groups in terms of sex, comorbidities, or symptom duration prior to hospitalization.
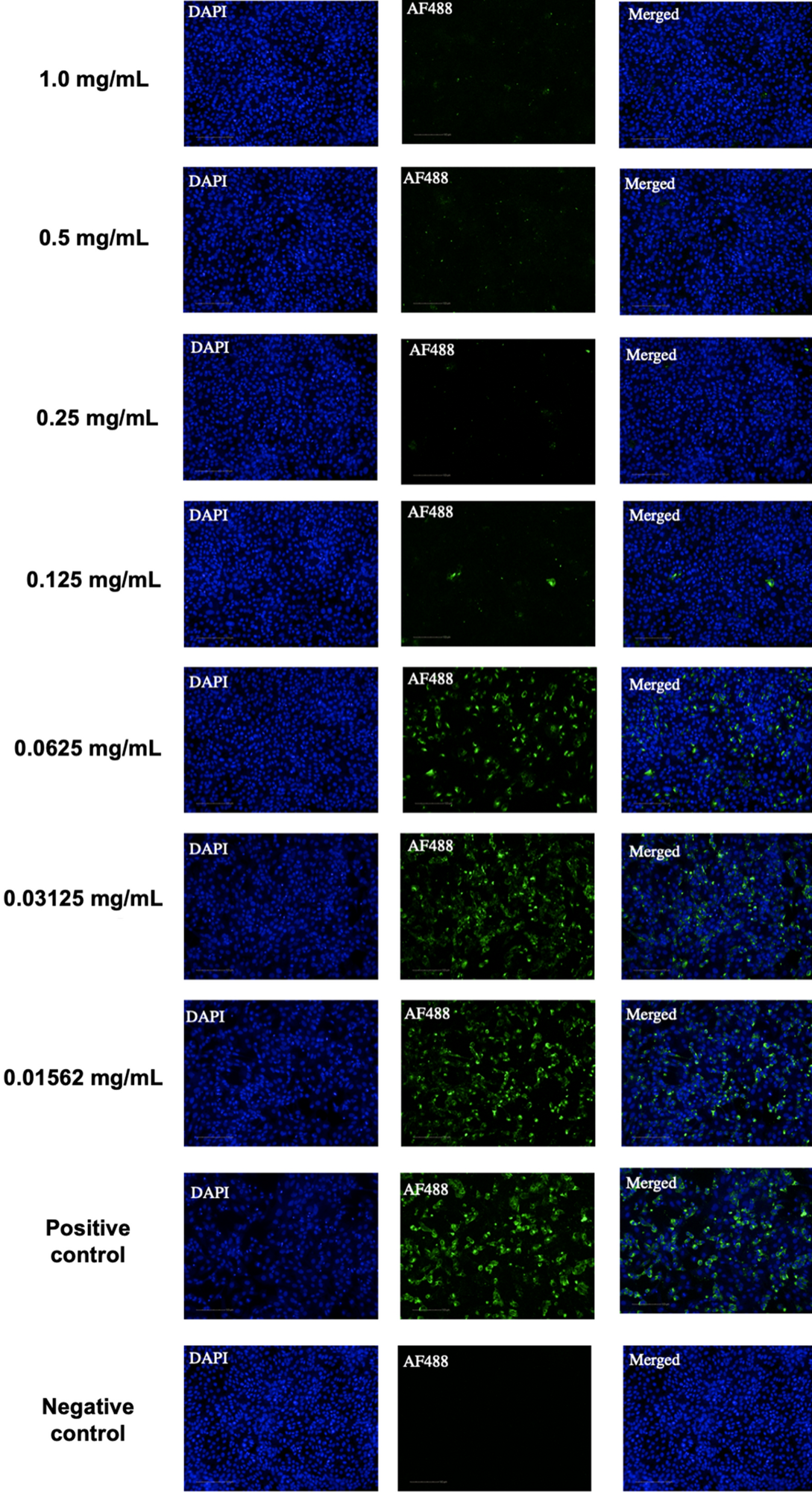 Indirect immunofluorescence (IIF) assay for the detection of SARS-CoV-2-infected cells. Representative images of the noncytotoxic concentrations (1.0 mg/mL up to 0.156 mg/mL) of APD observed with a 20 × objective. A mixture of MERS-CoV-infected and non-infected Vero cells were stained with convalescent serum monoclonal antibodies, followed by incubation with the Alexa488-conjugated goat anti-human IgG antibodies (green). Cells were counterstained with DAPI to stain the nuclei (blue). Positive (infected nontreated cells) and negative (noninfected cells) controls are shown at the bottom of the image. Images were taken using the Operetta High Content Imaging System (Perkin Elmer). Scale bar, 100 µm.
Indirect immunofluorescence (IIF) assay for the detection of SARS-CoV-2-infected cells. Representative images of the noncytotoxic concentrations (1.0 mg/mL up to 0.156 mg/mL) of APD observed with a 20 × objective. A mixture of MERS-CoV-infected and non-infected Vero cells were stained with convalescent serum monoclonal antibodies, followed by incubation with the Alexa488-conjugated goat anti-human IgG antibodies (green). Cells were counterstained with DAPI to stain the nuclei (blue). Positive (infected nontreated cells) and negative (noninfected cells) controls are shown at the bottom of the image. Images were taken using the Operetta High Content Imaging System (Perkin Elmer). Scale bar, 100 µm.
Implications for strategic planning
This study clearly showed that the mechanical action of the protocol that involves the mouthwash containing a compound with very specific antiviral effects against SARS-CoV-2 might reduce the symptoms of the patients and the subsequent spread of the virus.
“The outstanding results achieved in the active mouthwash group suggest that mouthwash in addition to other medications can be useful in the strategic planning of COVID-19 treatment by the World Health Organization”, emphasize the authors of this paper published in the journal Scientific Reports.
Nonetheless, disease dynamics, the healthcare environment, medications, pandemic stress, and sample size may represent possible study limitations. Hence, the interpretation and result generalizability should be performed with reservation, and further studies will be needed to contextualize these results in larger populations.