The coronavirus disease 2019 (COVID-19) pandemic has been caused by a novel coronavirus, namely, severe acute respiratory coronavirus 2 (SARS-CoV-2). As a result of the sustained efforts of scientists, we are now equipped with several vaccines to combat the pandemic.
In the UK, the Medicines and Regulatory Healthcare Agency (MHRA) recently licensed the antiviral drug molnupiravir for use in patients with mild-moderate COVID-19. While it is known that drugs are most effective when started early, the exact window in which drugs can be used remains elusive.
In a new study, published on the bioRxiv* preprint server, scientists aimed to determine the reaction of the molnupiravir parent drug (NHC) to different SARS-CoV-2 Variants of Concern (VoCs). They also sought to establish the therapeutic window in a human lung cell model.
 Study: Antiviral activity of molnupiravir precursor NHC against SARS-CoV-2 Variants of Concern (VOCs) and its therapeutic window in a human lung cell model. Image Credit: Quality Stock Arts / Shutterstock
Study: Antiviral activity of molnupiravir precursor NHC against SARS-CoV-2 Variants of Concern (VOCs) and its therapeutic window in a human lung cell model. Image Credit: Quality Stock Arts / Shutterstock

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Molnupiravir in the treatment of SARS-CoV-2 Infection
Molnupiravir is an antiviral pro-drug that was initially developed to counter the influenza virus. It is currently undergoing clinical trials in humans for the treatment of COVID-19.
Some preliminary results have indicated that the drug reduced the risk of hospitalization or death by 50%. It was further noted that the efficacy was unaltered by the timing of symptom onset, underlying risk factors, or a particular variant type.
Scientists have observed, in a ferret model, that treatment of SARS-CoV-2 infection with molnupiravir resulted in reduced upper respiratory tract viral load and also blocked transmission between animals.
The results were robust in a mice model as well, and a combination of molnupiravir and favipiravir was also found to be effective in a hamster model. However, most of these studies used an early variant of the virus rather than the more recent VOCs.
The parent drug of molnupiravir is known as NHC or ß-D-N4-hydroxycytidine. In two independent studies, the efficacy of NHC (against the Alpha and Beta variants in Vero E6 and Calu3 cells) has been demonstrated. However, the drug efficacy at inhibiting viral replication to the Delta variant has not been documented yet.
In the current study, scientists wished to fill this gap in the literature, and they used a human lung epithelial cell model (hACE2-A549 cells) to this end.
 In-vitro cytotoxicity of NHC to hACE2-A549 cells. Cytotoxicity of different concentrations (in µM) was measured using the Cell-titer Glo Assay to measure the percentage ATP production in treated cells compared to mock-treated cells (n=7). There was no significant difference in % ATP production of cells compared to control cells in most concentrations of NHC, except at 10uM (p<0.0001) and 0.1µM (p=0.02). To determine the inhibitory activity of NHC against different VOCs and an ancestral B-lineage virus, dose-response assays were performed by infecting hACE2-A549 cells at an MOI of 0.1 in media alone and in media containing 0.01, 0.1, 1, and 10µM NHC. After 72 hours incubation, cell supernatants were removed, and viral titers determined by plaque assay. The IC50 (the concentration of drug required to inhibit virus titer by 50%) was determined using non-linear regression with GraphPad Prism 9. The results demonstrated similar IC50 values for each variant and the ancestral strain of between 0.04 and 0.16µM concentrations.
In-vitro cytotoxicity of NHC to hACE2-A549 cells. Cytotoxicity of different concentrations (in µM) was measured using the Cell-titer Glo Assay to measure the percentage ATP production in treated cells compared to mock-treated cells (n=7). There was no significant difference in % ATP production of cells compared to control cells in most concentrations of NHC, except at 10uM (p<0.0001) and 0.1µM (p=0.02). To determine the inhibitory activity of NHC against different VOCs and an ancestral B-lineage virus, dose-response assays were performed by infecting hACE2-A549 cells at an MOI of 0.1 in media alone and in media containing 0.01, 0.1, 1, and 10µM NHC. After 72 hours incubation, cell supernatants were removed, and viral titers determined by plaque assay. The IC50 (the concentration of drug required to inhibit virus titer by 50%) was determined using non-linear regression with GraphPad Prism 9. The results demonstrated similar IC50 values for each variant and the ancestral strain of between 0.04 and 0.16µM concentrations.
Key Findings
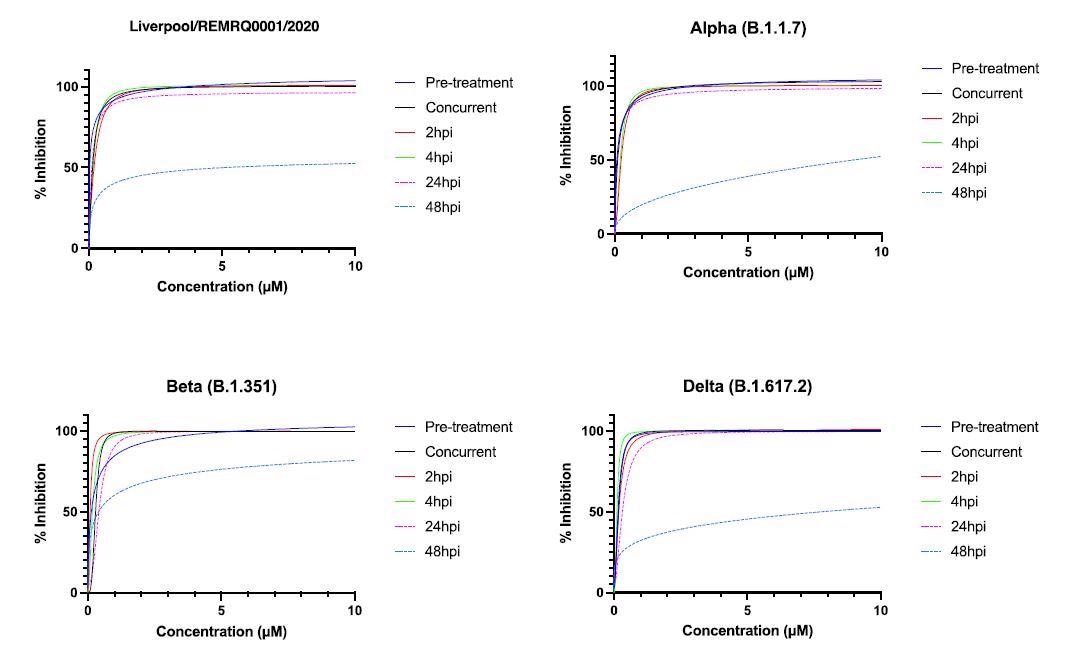
For this study, researchers performed dose-response assays (in parallel) to determine the IC50 (the concentration of drug 32 required to inhibit virus titer by 50%) of NHC, and this was done for different variants of SARS-CoV-2.
Human ACE-2 A549 cells were treated with NHC at different time points of time, i.e., either before, during, or after infection with SARS-CoV-2.
The active metabolite of molnupiravir is ß-D-N4-hydroxycytidine (NHC). In this study, researchers showed that ß-D-N4-hydroxycytidine (NHC) has similar activity against four variants of SARS-CoV-2 (B-lineage, Alpha, Beta, and Delta) in a human lung cell line.
The IC50 was found to be in the range of 0.04-0.16µM. Scientists also demonstrated that the activity of the drug began to drop after 48 hours post-infection.
The results obtained in this analysis support the clinical data showing an inhibitory effect of molnupiravir against SARS-CoV-2.
Molnupiravir mimics naturally occurring nucleosides to create error catastrophe during virus replication in a host. It is for this reason that it is expected to work against all variants of the virus.
This last claim was tested and proved on four variants, as mentioned earlier. The data are in line with the results of the MOVe-In trial, where the use of molnupiravir in hospitalized patients was stopped prematurely since a statistically significant effect was considered unlikely. It also supports the decision to license the drug for use in mild-moderate cases.
Limitations of in-vitro Systems
In-vitro studies have many limitations compared to live models of infection. The use of molnupiravir in a Syrian hamster model infected with SARS-CoV-2 resulted in a drop in viral load and reduced lung pathology. However, it was observed that treatment administered 12 hours post-infection was effective but not at 24 hours post-infection. More research is required to narrow down the ideal treatment window of the drug in humans with mild to moderate disease.
Conclusion
An advantage of molnupiravir over remdesivir is that it can be administered orally. However, it is vital to be cautious because antiviral resistance can develop quickly, as observed with the influenza antiviral Tamiflu.
A holistic analysis of the potential of SARS CoV-2 to develop resistance is necessary. Researchers have claimed that the treatment with molnupiravir could be most effective if used in combination with other therapies, such as those targeting a different part of the viral life cycle. This principle has been successfully applied in the treatment of HIV.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.