As the COVID-19 pandemic is still having a strong global impact, it is pivotal that countries maximize the health benefits of the available vaccine supplies based on context-specific COVID-19 epidemiology, transmission dynamics, and pre-existing immunity COVID-19 vaccine immunogenicity, efficacy, and safety.
 Study: Dosing interval strategies for two-dose COVID-19 vaccination in 13 low- and middle-income countries of Europe: health impact modelling and benefit-risk analysis. Image Credit: Spaxiax / Shutterstock
Study: Dosing interval strategies for two-dose COVID-19 vaccination in 13 low- and middle-income countries of Europe: health impact modelling and benefit-risk analysis. Image Credit: Spaxiax / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Vaccines currently on the market usually involve two doses with specific between-dose intervals, as informed by the clinical trials. More specifically, the time span between two vaccine doses is usually 3-4 weeks, although this may vary among different solutions.
Nonetheless, in practice, countries may utilize dosing intervals that are longer than recommended due to a plethora of different factors, most notably vaccine shortages, logistic constraints and administrative issues, but also comparable and potentially higher vaccine efficacy with the extended dosing interval.
To address this information gap, a research group from the London School of Hygiene & Tropical Medicine and Public Health England aimed to estimate the health impact and risk-benefit assessment of COVID-19 vaccination regarding different dosing intervals for low- and middle-income countries in Europe.
A stringent modeling approach
For that purpose, this research group used a dynamic transmission model that incorporated daily nationwide COVID-19 mortality in thirteen low- and middle-income countries, which are (alphabetically): Albania, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Bulgaria, Georgia, Republic of Moldova, Russian Federation, Serbia, North Macedonia, Turkey, and Ukraine.
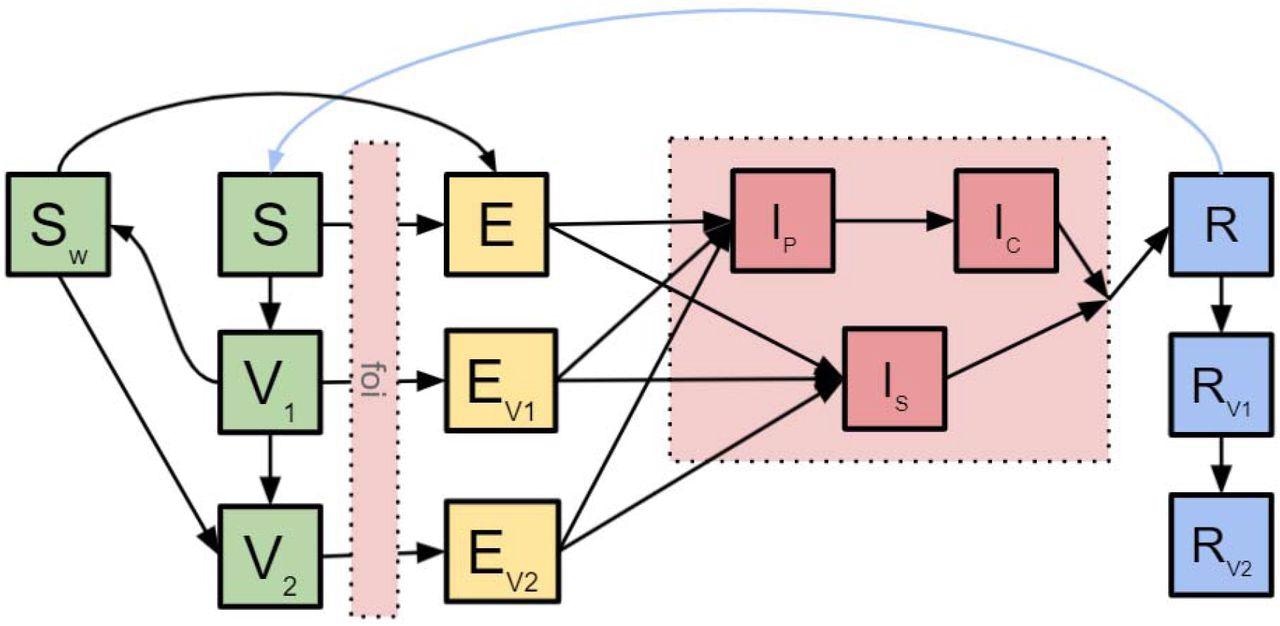 SARS-CoV-2 transmission dynamics and COVID-19 vaccination impact. The conceptual diagram describes the underlying mathematical models of SARS-CoV-2 transmission dynamics and COVID-19 vaccination impact. S - susceptible; V1 - individuals protected by the first dose only; V2 - individuals protected by both doses; Sw - individuals who have received their first dose but the protection has waned; E - exposed; Ev1 - exposed progressed from individuals in V1; Ev2 - exposed progressed from individuals in V2; Ip - pre-clinical infectious individuals; Ic - clinical individuals; Is - subclinical individuals; R - recovered; Rv1 - previously infected individuals whose infection-induced immunity has yet to wane and who have received the first dose; Rv2 individuals whose infection-induced immunity has yet to wane and who have received both doses.
SARS-CoV-2 transmission dynamics and COVID-19 vaccination impact. The conceptual diagram describes the underlying mathematical models of SARS-CoV-2 transmission dynamics and COVID-19 vaccination impact. S - susceptible; V1 - individuals protected by the first dose only; V2 - individuals protected by both doses; Sw - individuals who have received their first dose but the protection has waned; E - exposed; Ev1 - exposed progressed from individuals in V1; Ev2 - exposed progressed from individuals in V2; Ip - pre-clinical infectious individuals; Ic - clinical individuals; Is - subclinical individuals; R - recovered; Rv1 - previously infected individuals whose infection-induced immunity has yet to wane and who have received the first dose; Rv2 individuals whose infection-induced immunity has yet to wane and who have received both doses.
A vaccine similar to the Oxford-AstraZeneca COVID-19 (AZD1222) vaccine was used in the base case scenario, supplemented by sensitivity analyses regarding efficacies related to other COVID-19 vaccines. Furthermore, the scientists have used fixed dosing intervals at 4, 8, 12, 16, and 20 weeks and dose-specific intervals that prioritize specific doses for specific age groups.
Finally, the emergence of variants of concern has been accounted for in the model with adequate sensitivity analyses. At the same time, a risk-benefit assessment was conducted in order to quantify the trade-off between any harm caused by adverse events and health benefits after immunization procedure.
An enormous efficacy of longer dosing intervals
This study revealed that optimal strategies are, in fact, those that prioritize the first doses among adults older than 60 years of age, or those between 20-59 years of age, which was valid for 12 out of the 13 countries. More importantly, these strategies resulted in dosing intervals longer than six months, as a 4-week fixed dosing interval may give rise to 10.2% more fatal outcomes.
There was also a negative association between dosing interval and COVID-19 mortality within the investigated range. In comparison to risks, benefits were highest for strategies that included 8-12 weeks intervals, following the age-dependent rates of prevented deaths from COVID-19.
Although the researchers could not extend the modeling further into 2021 due to data availability issues, the available results point towards a sizeable efficacy of longer dosing intervals; however, the paper highlights how extraordinarily long dosing intervals should be avoided.
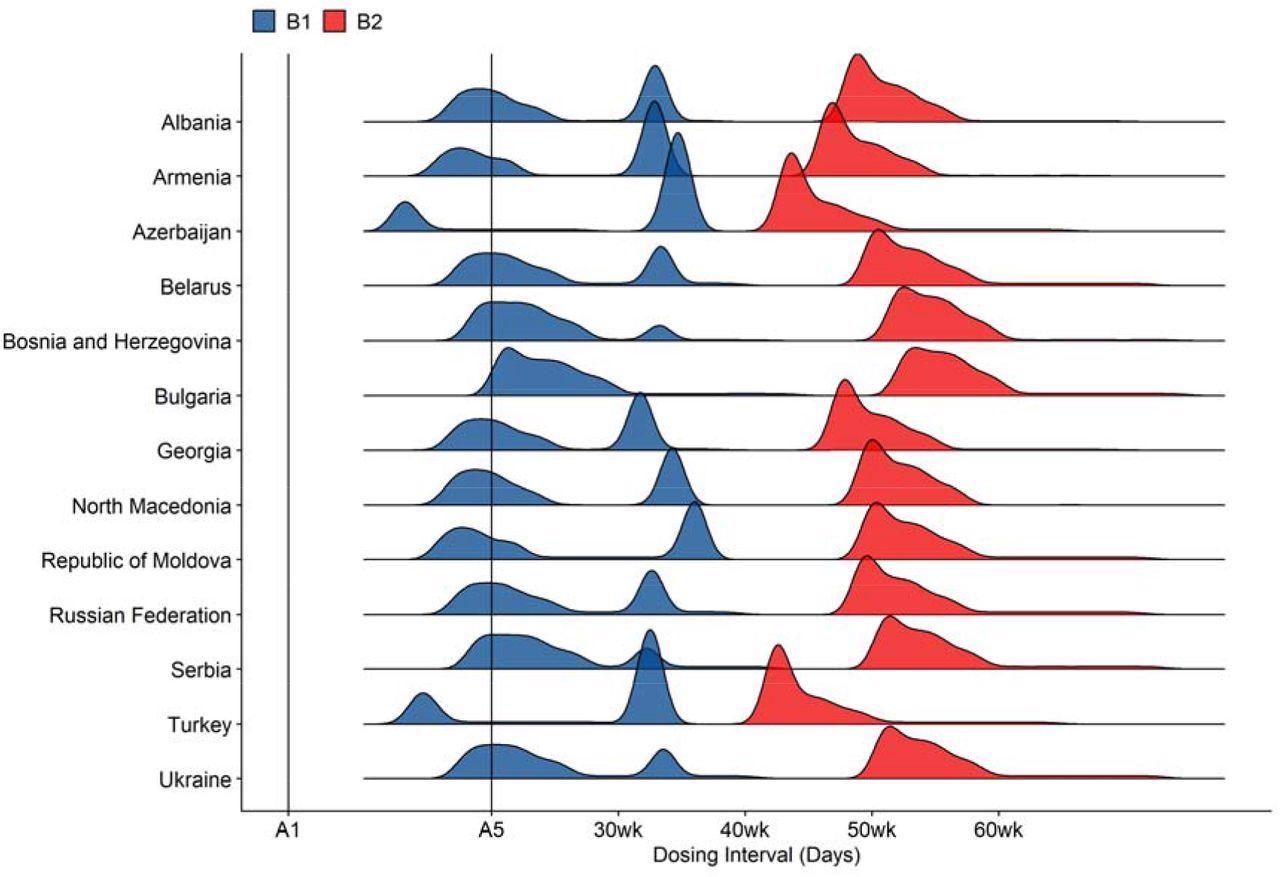 COVID-19 dosing intervals under strategies B1 and B2. These dosing strategies do not prescribe fixed dosing intervals. Vaccine allocations depend on whether or not coverage goals have been met in certain target groups. The distributions are outputs from dose allocation algorithms that capture such conditional relationships. Refer to Table 1 for descriptions of vaccination strategies.
COVID-19 dosing intervals under strategies B1 and B2. These dosing strategies do not prescribe fixed dosing intervals. Vaccine allocations depend on whether or not coverage goals have been met in certain target groups. The distributions are outputs from dose allocation algorithms that capture such conditional relationships. Refer to Table 1 for descriptions of vaccination strategies.
Benefits for societies around the world
In any case, even though dosing intervals of over six months are significantly longer than the current label recommendation for most vaccines, this approach could reduce COVID-19 mortality in low- and middle-income countries in Europe. Still, it has to be noted that certain vaccine features (such as fast waning of first doses) significantly shorten the optimal dosing intervals.
“The results we have observed are robust to different vaccine characteristics assumptions of vaccine efficacy estimates versus dosing interval relationships,” further emphasize the authors of this medRxiv paper. “Including additional outcomes and benefits for society is expected to lead to similar results,” they add.
Since countries that were included in this study have diverse demographics and age structures, contact patterns and historical outbreak experiences histories, the overall conclusions can be valuable in decision-making in low- and middle-income countries around the world.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Liu, Y. et al. (2021). Dosing interval strategies for two-dose COVID-19 vaccination in 13 low- and middle-income countries of Europe: health impact modelling and benefit-risk analysis, medRxiv, https://doi.org/10.1101/2021.11.27.21266930, https://www.medrxiv.org/content/10.1101/2021.11.27.21266930v1
- Peer reviewed and published scientific report.
Liu, Yang, Carl A.B. Pearson, Frank G. Sandmann, Rosanna C. Barnard, Jong-Hoon Kim, Stefan Flasche, Mark Jit, and Kaja Abbas. 2022. “Dosing Interval Strategies for Two-Dose COVID-19 Vaccination in 13 Middle-Income Countries of Europe: Health Impact Modelling and Benefit-Risk Analysis.” The Lancet Regional Health - Europe, April, 100381. https://doi.org/10.1016/j.lanepe.2022.100381. https://www.sciencedirect.com/science/article/pii/S2666776222000746?via%3Dihub.