The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOC) are now co-circulating globally with the parental wild-type strain that first emerged in China in 2019. SARS-CoV-2 VOCs have become predominant in countries or regions with high virus circulation and low vaccine coverage rates.
The World Health Organization (WHO) recognizes VOCs as variants of SARS-CoV-2 for which there is evidence of increased transmissibility, higher severity of disease, evasion from antibodies generated during previous infection or vaccination, or detection failures during diagnostic testing. Subsequently, these VOCs are responsible for escalating the coronavirus disease 2019 (COVID-19) cases globally.
The Delta variant (B.1.617.2) of SARS-CoV-2, which first emerged in India in October 2020, is twice as contagious as other VOCs. This variant is now circulating in over 96 countries, fueling outbreaks in regions that had previously been able to suppress SARS-CoV-2 transmission and thus causing a resurgence in regions with highly vaccinated populations.
 Study: SARS-CoV-2 Delta variant saliva viral load is 15-fold higher than wild-type strains. Image Credit: Andrey_Popov / Shutterstock.com
Study: SARS-CoV-2 Delta variant saliva viral load is 15-fold higher than wild-type strains. Image Credit: Andrey_Popov / Shutterstock.com

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Study design
In a recent study published on the medRxiv* preprint server, a team of Japanese researchers hypothesized that a higher viral load of SARS-CoV-2 Delta variants in saliva compared to their parental wild-type strains contributes to the high transmissibility of these variants. Saliva transmits SARS-CoV-2 infection via three routes including droplets, contact, and aerosol or air droplets. Furthermore, saliva contains infectious virions released from the oral epithelia and the salivary glands.
The researchers used the real-time quantitative polymerase chain reaction (RT-qPCR) assay to determine viral load as viral copy number of 22 and 32 genetically confirmed saliva samples that were found to be positive for the SARS-CoV-2 wild-type and Delta strains, respectively. They used a qPCR protocol provided by the National Institute of Infectious Diseases of Japan.
In conjunction with this, the researchers synthesized viral ribonucleic acid RNA and measured the RNA levels of the variant and wild-type strain samples, as well as for the whole saliva and centrifugal supernatant in each group, using Poisson's null distribution equation.
Whole saliva samples were collected from the visitors of a fever outpatient clinic in Nagoya, Japan. These samples were taken at two different time points, the first was between November and December 2020 before the emergence of the Delta variant and the second between August and September 2021 after its emergence. The Bio Medical Laboratories Inc. (BML) in Tokyo conducted diagnostic tests on these samples.
The researchers compared the viral copy numbers of the variant and wild-type strains statistically using the Mann-Whitney’s U-test or Wilcoxon’s signed-rank test. They also investigated whether virions were associated with host cells or floating away from them. The proportion of host cells isolated with human chromosome-specific qPCR through centrifugation was 99.5%.
A 15-fold higher viral load in the saliva of Delta-variant infected individuals
When comparing the whole saliva of patients infected with the wild-type SARS-CoV-2 strain and those infected with Delta variants, the viral loads were significantly different. The median copy number of the Delta variant in 1 ml of the whole saliva, as compared to the wild-type strains, was 15.1 times higher.
In addition, the viral load was 15.9 times and 4.2 times higher in the whole saliva compared to its value in the pertinent supernatant for Delta-variants and wild-type strains, respectively. These findings indicate that most of the viruses in the whole saliva samples are associated with the host cells.
Several previous studies have reported this kind of comparison based on the estimation of viral copy numbers. The viral copy number is an estimation of the number of copies of a gene in the genome of an organism. For SARS-CoV-2, the viral copy number is an estimation of copies of RNA.
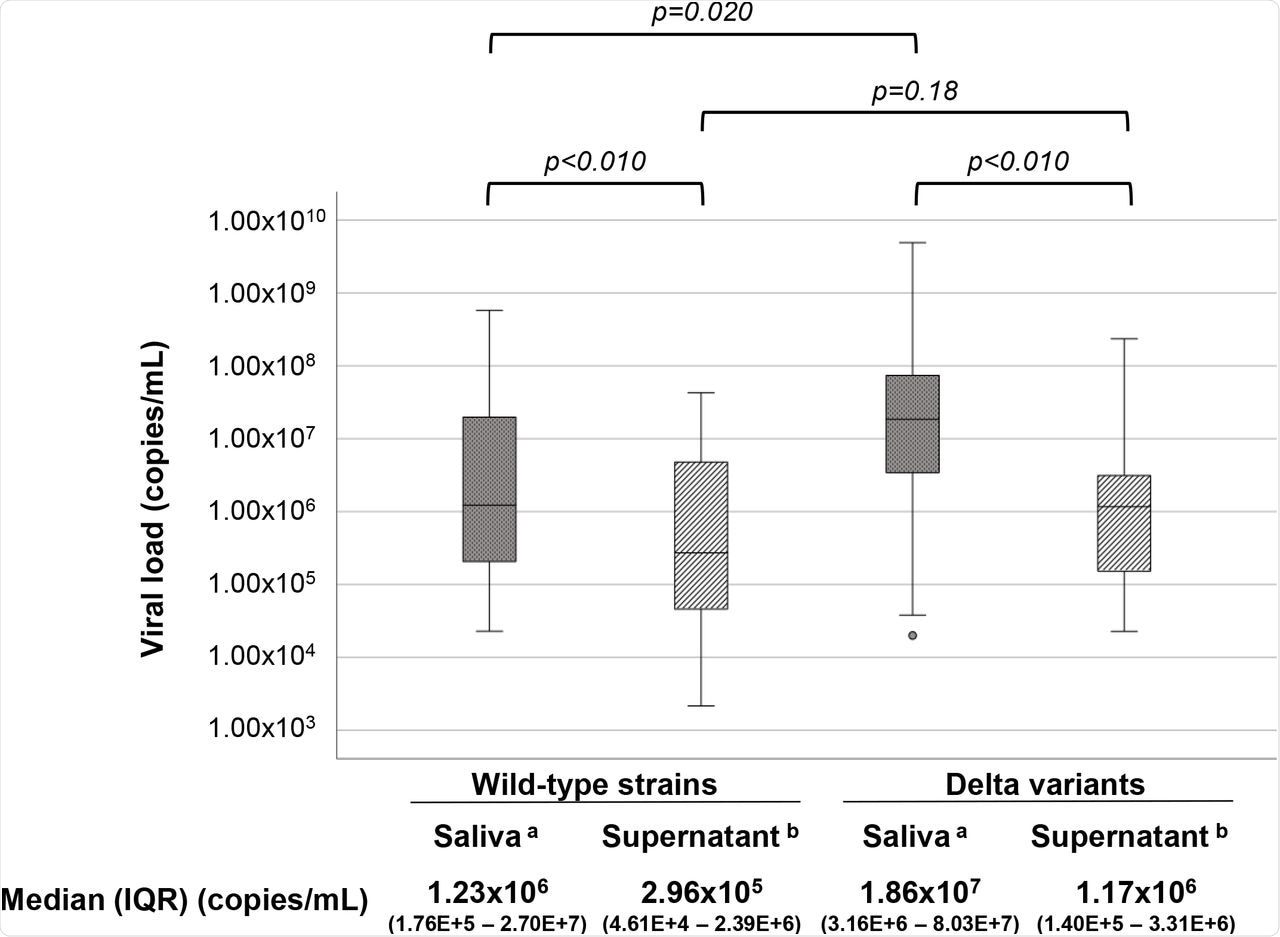 Comparisons between wild-type strains and Delta variants and between whole saliva and pertinent supernatant were determined using Mann–Whitney’s U-test and Wilcoxon’s signed-rank test, respectively. Viral load was significantly lower in saliva supernatant than whole saliva for both strains (p = 1.2E-5 for Delta variants and p = 6.6E-3 for wild-type strains). Delta variant’s viral load was higher than the wild-type strain in whole saliva (p = 0.020). Viral loads of both variants and wild-type strains in whole saliva were significantly higher (15.9 times and 4.2 times, respectively; p = 1.2E-5 and p = 6.6E-3, respectively) than the pertinent supernatant. Delta variant’s viral load in the supernatant was 4.0 times higher (1.17E+6) but not significant (p = 0.18) than wild-type strains (2.96E+5).
Comparisons between wild-type strains and Delta variants and between whole saliva and pertinent supernatant were determined using Mann–Whitney’s U-test and Wilcoxon’s signed-rank test, respectively. Viral load was significantly lower in saliva supernatant than whole saliva for both strains (p = 1.2E-5 for Delta variants and p = 6.6E-3 for wild-type strains). Delta variant’s viral load was higher than the wild-type strain in whole saliva (p = 0.020). Viral loads of both variants and wild-type strains in whole saliva were significantly higher (15.9 times and 4.2 times, respectively; p = 1.2E-5 and p = 6.6E-3, respectively) than the pertinent supernatant. Delta variant’s viral load in the supernatant was 4.0 times higher (1.17E+6) but not significant (p = 0.18) than wild-type strains (2.96E+5).
Conclusions
According to the authors, this is the first study that determines the Delta variant’s load in whole saliva samples. The implications of the results discussed here are crucial in preventing the spread of SARS-CoV-2 infection.
The current study estimates that the viral load of the SARS-CoV-2 Delta variant in the saliva is 15 times more than the parent or wild-type strain. Such a high viral load indicates the presence of large quantities of virions in the saliva of a variant-infected individual.
The variants’ median number of 1.17 x 106 virions per milliliter in the supernatant suggests that aerosols generated by variant-infected individuals contained more than one million virions per milliliter. Taken together, these findings explain the higher transmissibility of all VOCs, especially the Delta variant, and the significantly higher increase in the number of COVID-19 cases following the emergence of the Delta variant.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.