Several variants of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) have emerged during the present coronavirus disease 2019 (COVID‐19) pandemic complicating the development of prophylactic therapy for COVID-19. Further, several strains of common-cold coronaviruses (HCoVs) have been identified which are known to be endemic in humans. Evidence exists about the cross-reactivity of antibodies against spike glycoprotein (S) belonging to various coronaviruses.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
It remains to be explored if antibody responses resulting from cross-reactivity, elicited by vaccinations or natural infections specifically aimed at the S2 subunit may confer protection against SARS-CoV-2 and its variants. Evasion of the immune response by the variants of concern (VOC) is attributed to the amino acid substitutions on the S1 subunit which is considered to be less conserved when compared to the S2 subunit. Monoclonal antibodies specific for the S2 subunit have been isolated during the present SARS-CoV-2 and previous SARS-CoV pandemics indicating the possibility of targeting the S2 subunit for broad-spectrum protection against various coronaviruses (CoVs).
The research, released as a preprint on the bioRxiv* server, attempts to explore the contribution of humoral immunity targeting SARS-CoV-2 S2 in conferring protection against SARS-CoV-2 and its variants.
Background
The scientists in the present study attempted to assess the cross-reactivity between HCoV S and SARS‐CoV‐2 S. They immunized C57BL/6J mice with HCoV‐OC43 S using a DNA vaccine that was administered as two doses each 4 weeks apart.
It was observed that the prime dose of the vaccine did not elicit a strong immune response, however, the booster dose of the vaccine produced a strong immune response and elevated levels of antibodies against the SARS‐CoV‐2 receptor-binding domain (RBD), spike protein S1 subunit (S1) or spike protein S2 subunit (S2). There was a 2.5 times increase in the antibodies against SARS‐CoV‐2 S2.
Serum from mice immunized with HCoV-043 S exhibited neutralizing activity against retroviral vectors pseudotyped with SARS‐CoV‐2 S and this was found to be lower than the response against homologous HCoV‐OC43 S. Further, plaque reduction neutralization test (PRNT) revealed that serum from HCoV- OC43 S immunized mice exhibited weak neutralization activity when tested on authentic SARS‐CoV‐2 Wuhan and Alpha strains. Notably, this effect was not observed in all of the immunized mice and it was observed only at the highest concentrations of the serum tested.
Further, cross-reactivity between HCoV S and SARS‐CoV‐2 S was also assessed by evaluating if one S variant is capable of priming the responses to another variant. C57BL/6J mice were previously immunized with a prime dose of HCoV‐OC43 S and subsequently immunized with SARS‐CoV‐2 Wuhan S.
The control mice received both prime and booster doses of the same vaccine (HCoV‐OC43 S alone or SARS‐CoV‐2 Wuhan S) or received PBS and one subsequent dose of SARS‐CoV‐2 Wuhan S.
In mice that received a prime dose of HCoV‐OC43 S, a subsequent single dose of SARS‐CoV‐2 Wuhan S elicited 1.9 times higher levels of antibodies against SARS‐CoV‐2 S2 which was a strong response when compared to the other groups.
Previous HCoV‐OC43 S immunization significantly elevated the IgG antibody levels elicited by subsequent single-dose vaccination with SARS‐CoV‐2 Wuhan S and additionally, the IgG antibodies exhibited binding to natural SARS‐CoV‐2 S when tested in cell-based assays.
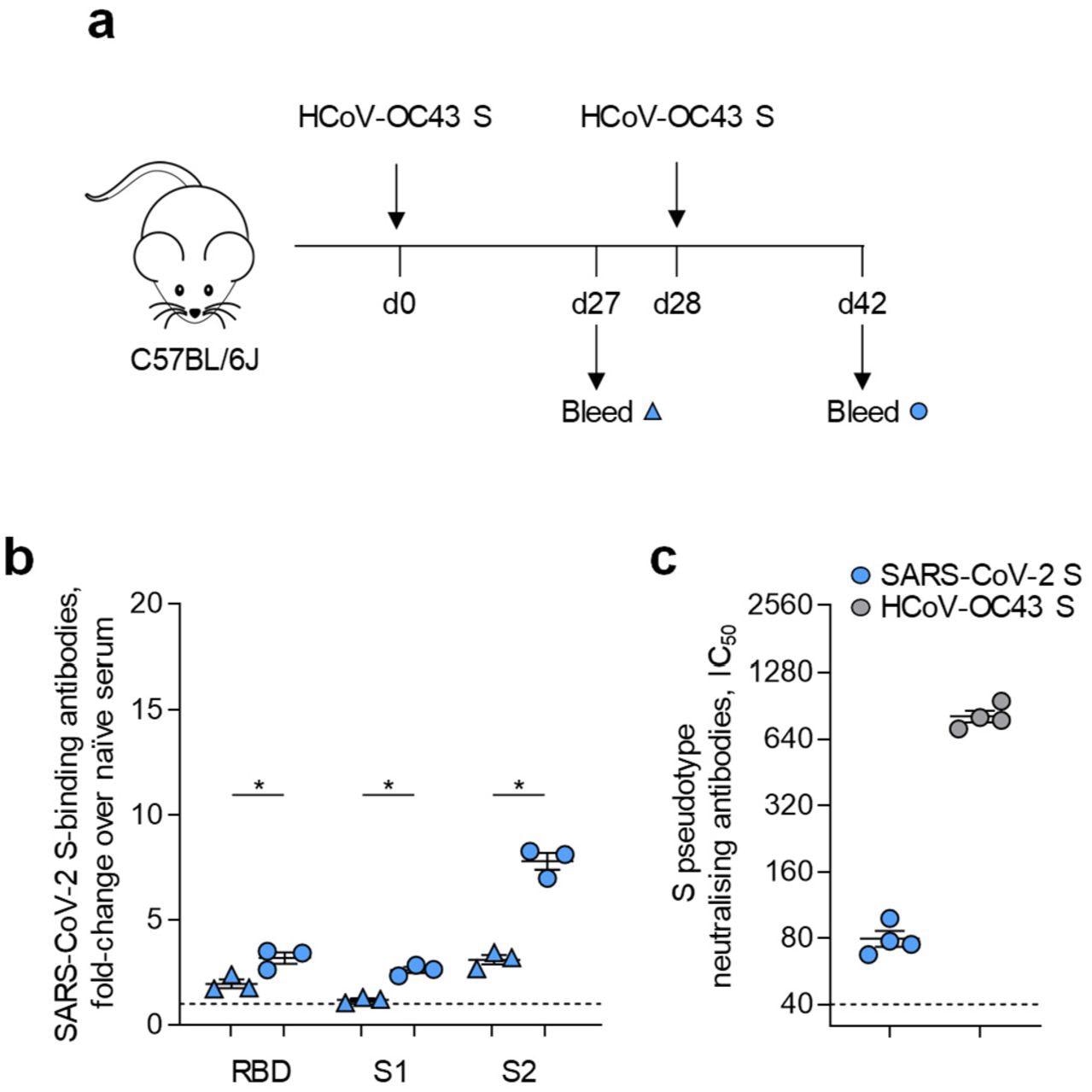 Prior HCoV-OC43 S immunity cross-reacts with SARS-CoV-2 S. A, Diagram of HCoV-OC43 S immunization regimen and serum sample collection. B, Levels of ELISA detected antibodies reacting with SARS-CoV-2 RBD, S1 or S2 in C57BL/6J mice (n=3) after one or two doses of an HCoV-OC43 S DNA vaccine. P values were calculated with paired Student’s t-tests. C, Levels (IC50) of antibodies in the sera of mice after two doses of an HCoV-OC43 S DNA vaccine (n=4), compared with sera from unvaccinated mice (n=4), able to neutralize retroviral vectors pseudotyped with HCoV-OC43 S or SARS-CoV-2 S. Values in B and C are from two different experiments.
Prior HCoV-OC43 S immunity cross-reacts with SARS-CoV-2 S. A, Diagram of HCoV-OC43 S immunization regimen and serum sample collection. B, Levels of ELISA detected antibodies reacting with SARS-CoV-2 RBD, S1 or S2 in C57BL/6J mice (n=3) after one or two doses of an HCoV-OC43 S DNA vaccine. P values were calculated with paired Student’s t-tests. C, Levels (IC50) of antibodies in the sera of mice after two doses of an HCoV-OC43 S DNA vaccine (n=4), compared with sera from unvaccinated mice (n=4), able to neutralize retroviral vectors pseudotyped with HCoV-OC43 S or SARS-CoV-2 S. Values in B and C are from two different experiments.
A high-throughput WHO-benchmarked assay was further performed and it showed that immunization with HCoV‐OC43 S prior to a single dose of SARS‐CoV‐2 Wuhan S vaccine-induced neutralizing antibodies against authentic SARS‐CoV‐2 Wuhan strain, D614G strains, and other variants of concern tested in the study. This effect was not observed in mice that received a single dose of SARS‐CoV‐2 Wuhan S vaccine alone.
The findings from this study demonstrated that prior exposure to HCoV‐OC43 S amplifies the response elicited by subsequent SARS‐CoV‐2 S exposure. Prior HCoV‐OC43 S exposure has the potential for transforming the nature of SARS‐CoV‐2 S exposure from subimmunogenic to immunogenic.
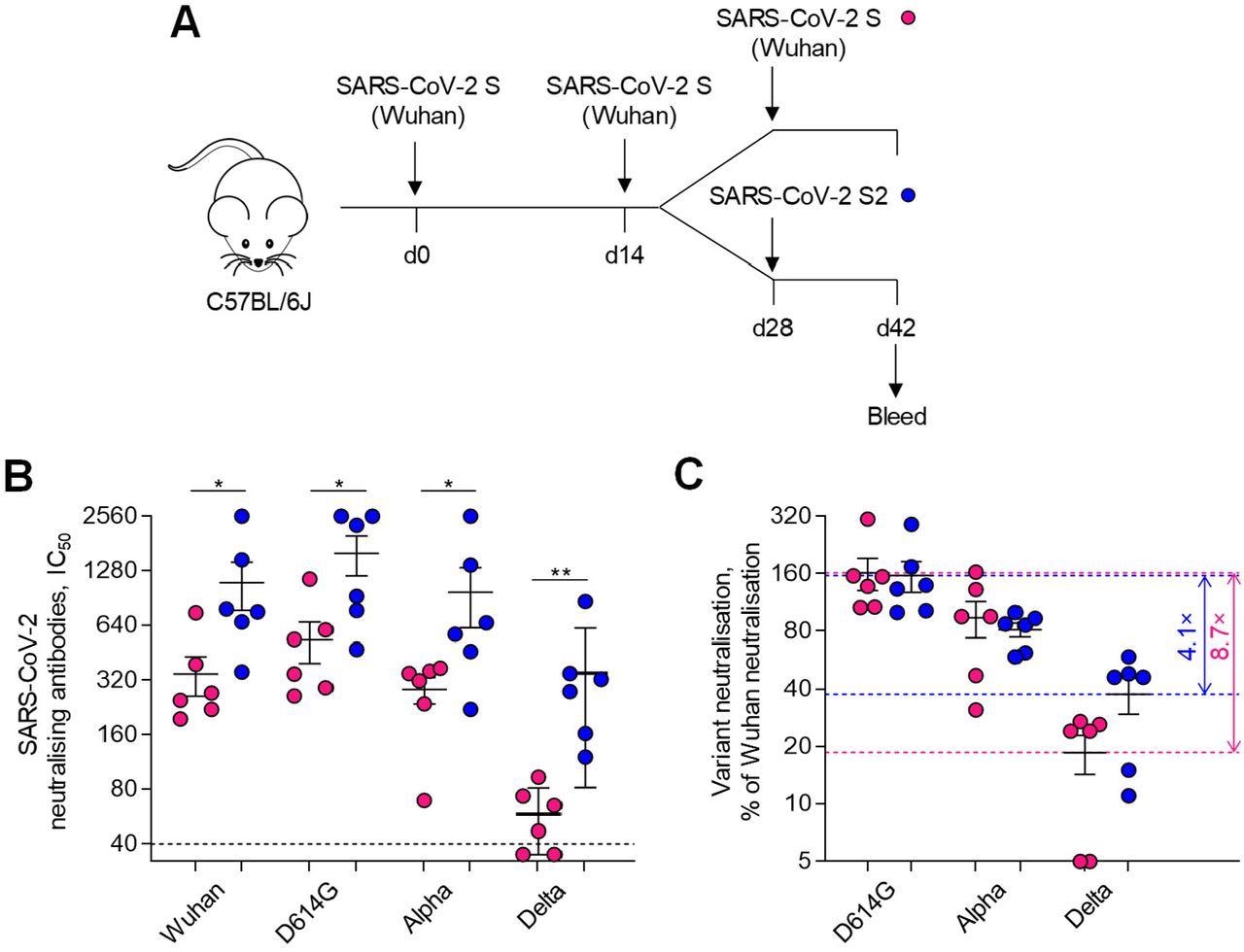 SARS-CoV-2 S2 immunisation boosts prior SARS-CoV-2 S-induced cross-reactivity. A, Diagram of SARS-CoV-2 full-length S and S2 immunisation regimens and serum sample collection. B, Levels (IC50) of antibodies able to neutralise the indicated authentic SARS-CoV-2 strains and VOCs in the sera of mice that were immunised twice with SARS-CoV-2 full-length S and then received a third immunisation with either SARS-CoV-2 full-length S or S2 (n=6 per group). C, Neutralisation activity against heterologous SARS-CoV-2 strains and VOCs, expressed as a percentage of neutralisation of the homologous Wuhan strain, in the sera of the same mice. Horizontal dashed lines mark the average neutralising activity for the maximally and minimally neutralised group and numbers represent the ratio between the two.
SARS-CoV-2 S2 immunisation boosts prior SARS-CoV-2 S-induced cross-reactivity. A, Diagram of SARS-CoV-2 full-length S and S2 immunisation regimens and serum sample collection. B, Levels (IC50) of antibodies able to neutralise the indicated authentic SARS-CoV-2 strains and VOCs in the sera of mice that were immunised twice with SARS-CoV-2 full-length S and then received a third immunisation with either SARS-CoV-2 full-length S or S2 (n=6 per group). C, Neutralisation activity against heterologous SARS-CoV-2 strains and VOCs, expressed as a percentage of neutralisation of the homologous Wuhan strain, in the sera of the same mice. Horizontal dashed lines mark the average neutralising activity for the maximally and minimally neutralised group and numbers represent the ratio between the two.
Vaccine broad-spectrum neutralizing activity
The scientists further evaluated the neutralizing potential of S2-targeting antibodies against SARS-CoV-2 and other CoVs. C57BL/6J mice were immunized with either a DNA vaccine that encodes membrane-bound SARS-CoV-2 subunit or with recombinant monomeric SARS‐ CoV‐2 S2 protein or with a DNA vaccine encoding the full‐length SARS‐CoV‐2 S.
S2-specific ELISA performed revealed that immunization with DNA vaccines encoding membrane‐bound SARS‐CoV‐2 S2 subunit or with recombinant monomeric SARS‐ CoV‐2 S2 protein elicited 4 fold higher levels of antibodies targeting the S2 subunit when compared to the DNA vaccine encoding the full length S protein. This suggests that full length S protein vaccine is able to elicit only a partial response against the S2 subunit. Notably, antibodies against the S2-DNA vaccine recognized the intact SARS‐CoV‐2 S in cell-based assays while antibodies elicited by recombinant S2 protein did not. This indicated that the S2 construct encoded by the DNA vaccine retains the epitopes for recognizing full-length S.
Studies with peptide arrays spanning the SARS-CoV-2 S2 subunit showed that the mouse sera from SARS-CoV-2 immunized mice reacted similarly to the COVID‐19 convalescent human sera except in the case of epitopes in the fusion peptide region where the mouse sera showed less reactivity.
Flow cytometry analysis in a cell-based assay shows that antibodies induced by DNA vaccine encoding the SARS‐CoV‐2 S2 recognized the full-length S from the SARS-CoV-2 Wuhan strain and additionally from all four HCoVs, other SARS-CoV-2 strains and variants of concern (VOCs) tested, two bat CoVs, bat SARS‐like coronavirus WIV1 and bat coronavirus RaTG13.
Further, the antibodies exhibited broad neutralizing activity against retroviral vectors pseudotyped with all the CoV S proteins that were tested in this study. Similar to antibodies elicited by vaccination with full length S protein, the antibodies elicited by SARS‐CoV‐2 S2 immunization, neutralized SARS‐CoV‐2 Wuhan and D614G strains, as well as the VOCs that were tested.
The findings suggest that immunization with membrane-bound SARS‐CoV‐2 S2 elicited antibodies with broad-spectrum neutralizing activity against the animal and human CoVs.
Third booster dose implications
The scientists attempted to assess the extent of in vivo protection provided by SARS‐CoV‐2 S2 immunization. SARS‐CoV‐2 S2 vaccinated and control unvaccinated K18‐hACE2 transgenic mice expressing the human ACE2 receptor were infected with SARS‐CoV‐2 Wuhan or Alpha variants.
The infected mice were evaluated for the expression levels of SARS‐CoV‐2 envelope (E) RNA in their lungs. It was observed that lungs of unvaccinated mice had detectable levels of SARS‐CoV‐2 E RNA which was not observed in SARS‐CoV‐2 S2 vaccinated mice. This indicates the protective effect of SARS‐CoV‐2 S2 vaccination against SARS‐CoV‐2 Wuhan and Alpha challenge which corroborates the findings from the in vitro neutralization studies.
Most COVID-19 vaccines are administered as a two-dose regime and currently, a third dose is also being recommended. Booster doses of homologous vaccines are known to amplify overall antibody titers. However, there is no conclusive evidence on their cross-reactivity with heterologous strains. The study further explored SARS‐CoV‐2 S induced cross-reactivity by administering two doses of SARS‐CoV‐2 S Wuhan vaccine to C57BL/6J mice and a third subsequent dose of either the same vaccine or a different vaccine against SARS‐CoV‐2 S2.
It was found that two weeks after the third dose of vaccines, the sera collected from all mice exhibited significant neutralization activity against the SARS-CoV-2 Wuhan strain, D614G strain, and Alpha VOC.
Notably, the sera collected from mice that received the SARS‐CoV‐2 S2 vaccine as the third dose exhibited higher neutralization activity compared to the full length S vaccine which was prominently seen in the case of Delta VOC.
The findings indicate that immunization with SARS‐CoV‐2 S2 may elicit a strong immune response against SARS-CoV-2 VOC when given as a third booster dose after immunization with two doses of SARS‐CoV‐2 full‐length S.
Conclusion
The present study demonstrates the potential of the S2‐targeting vaccine to render broad-spectrum protection against SARS‐CoV‐2 and its variants. In mice that received prior HCoV‐OC43 S vaccination, the antibody response elicited by subsequent doses of SARS‐CoV‐2 S was amplified especially against SARS‐CoV‐2 S2. Further, vaccination of mice with S2‐based vaccine-elicited antibodies exhibited potent in vitro neutralization activity against all the human and animal CoVs tested in the study. Additionally, the vaccine also conferred in vivo protection against SARS‐CoV‐2 in mice. It elicited a strong immune response when given as a third booster dose after vaccination with SARS‐CoV‐2 full‐length S. SARS-CoV-S2 may be a potential target for developing a pan-CoV vaccine which may provide an effective broad-spectrum prophylactic therapy during the present and future pandemics caused by CoV infections.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources