Vaccines against the coronavirus disease 2019 (COVID-19) were developed at an unprecedented speed, which was followed by robust mass vaccination campaigns against COVID-19 worldwide. However, the emergence of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants threatens to reverse the progress made thus far in limiting the spread of this deadly viral infection. As the SARS-CoV-2 continues to wreak havoc across the world, the need for the rapid generation of more efficient vaccines is crucial.
 Study: SARS-CoV-2 Virus-like Particles produced by a single recombinant baculovirus generate potent neutralizing antibody that protects against variant challenge. Image Credit: Design_Cells / Shutterstock.com
Study: SARS-CoV-2 Virus-like Particles produced by a single recombinant baculovirus generate potent neutralizing antibody that protects against variant challenge. Image Credit: Design_Cells / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study design
In a recent study published on the bioRxiv* preprint server, a team of researchers developed and tested a virus-like particle (VLP) for SARS-CoV-2, based on the co-expression of the spike (S), membrane (M), and envelope (E) structural proteins using the baculovirus expression system. VLP vaccine technology enables the production of virus-like mimics that can stimulate strong antibody responses in the host cells based on the expression of the structural proteins of a target virus.
The researchers constructed a recombinant baculovirus driving the expression of SARS-CoV-2 S, M, and E proteins. They also developed individual recombinant viruses expressing S, M, or M+E proteins. Spodoptera frugiperda (Sf9) cells were then infected with each of these recombinant viruses, followed by immunoblotting at three days post-infection to confirm the expression of proteins in these cells.
Evaluating the antigenicity and immunogenicity of SARS-CoV-2 VLPs
The recombinant viruses expressing only S protein and the VLPs displayed two bands that were identified with an S-specific antiserum. The recombinant baculovirus system showed a single band, thereby suggesting that they alone could produce all the structural proteins required for SARS-CoV-2 VLP formation. Purified VLPs formed a distinct band at about 35% sucrose on purification, the peak fraction of which reacted most strongly with anti-S monoclonal antibody CR3022.
Electron microscopy (EM) imaging showed that VLPs with requisite coronavirus structural proteins assemble into vesicle-like structures. The EM analysis of purified VLPs showed heterogeneous vesicles with a diameter of about 100 nanometers (nm) and a distinct fringe of the depth of about 10 n that resembled the crown-like spikes defining coronavirus morphology.
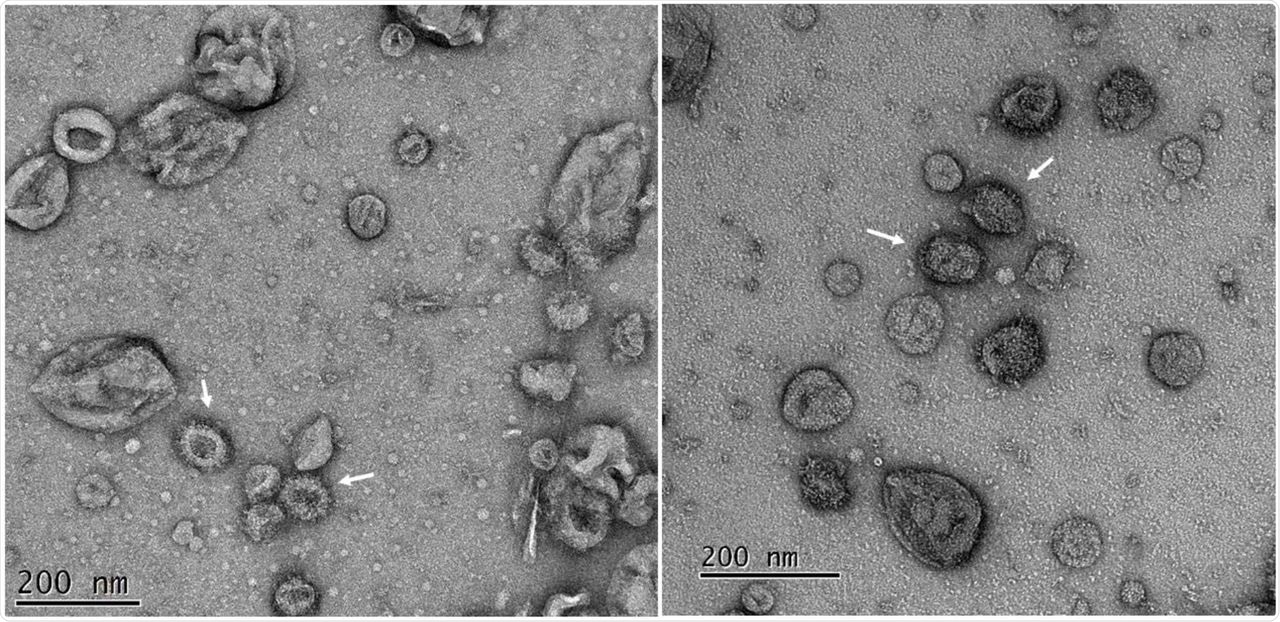
Transmission electron microscopy of purified SARS-CoV-2 VLPs purified from insect cells. Fractions were diluted to reduce the sucrose and re-concentrated before adsorption to carbon-coated grids. The grids were strained with 2% uranyl acetate. Peak fractions (cf. Figure 3) of the VLPs showed multiple vesicle-like structures among which are many with fringe-like projections on their surface (arrowed). Two typical fields of the same adsorbed sample are shown.
Next, the researchers assessed the antigenicity of insect cell-derived SARS-CoV-2 VLPs. VLPs present in the peak fraction during EM analysis were coated to enzyme-linked immunosorbent assay (ELISA) plates and probed with antibody-positive convalescent sera. Multiple convalescent sera, but not the serum controls, reacted strongly with immobilized VLPs. These results showed that SARS-CoV-2 VLPs were strongly antigenic.
Furthermore, the researchers tested the immunogenicity of these insect cell-derived VLPs bearing the SARS-CoV-2 S protein to demonstrate them as a candidate vaccine using the Syrian hamster as an animal model. Syrian hamsters infected with SARS-CoV-2 showed lung pathology similar to that reported in COVID-19 patients and had a neutralizing antibody response that protected them from subsequent infection.
Syrian hamsters were vaccinated with gradient purified VLPs at a dosage of 10 mg per immunization and were given booster doses four weeks after the priming dose. Seroconversion and neutralizing antibodies were observed in four out of five animals after the prime dose in the immunized group, which protected them against subsequent variant live virus challenge. Five additional control animals that received no treatment showed zero neutralizing activity.
 Neutralizing antibody development in immunized hamsters. Groups of Syrian hamsters were immunized with two doses of VLP candidate vaccine or untreated as controls and their sera tested for S binding using an RBD competition ELISA as described. A – Prior to live virus challenge. B - Post-virus challenge. The dates of immunization and challenge are shown. Two animals, one from each group, culled two days after challenge are indicated at day 44 in the right panel. C – Serum titers to RBD determined in the two animals identified in B at the time of sacrifice.
Neutralizing antibody development in immunized hamsters. Groups of Syrian hamsters were immunized with two doses of VLP candidate vaccine or untreated as controls and their sera tested for S binding using an RBD competition ELISA as described. A – Prior to live virus challenge. B - Post-virus challenge. The dates of immunization and challenge are shown. Two animals, one from each group, culled two days after challenge are indicated at day 44 in the right panel. C – Serum titers to RBD determined in the two animals identified in B at the time of sacrifice.
Conclusions
To conclude, this study presents a feasible vaccine candidate against SARS-CoV-2, which should be strongly considered for large-scale production and clinical assessment. The SARS-CoV-2 VLP designed in this study was found to be immunogenic in the absence of an adjuvant and generated a serum response in all immunized animals, which was further boosted following the live virus challenge.
Subsequently, immunized animals demonstrated reduced weight loss and recovered from an infection faster than the control animals. There were no adverse outcomes or deaths reported in the vaccinated animals following immunization.
VLPs were administered parenterally in animals and the live virus was administered directly to mucosal surfaces in the nasal passage during testing. It was observed that the mucosal surface antibody levels were inadequate to prevent infection in the immunized animals, although the systemic immune response generated in these animals was adequate to restrict virus proliferation and reduce the severity of the disease.
Taken together, these results indicate that SARS-CoV-2 VLPs are a viable vaccine candidate. Most importantly, these VLPs were produced using a technology that has been demonstrated as scalable in several past studies.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sullivan, E., Sung, P., Wu, W., et al. (2021). SARS-CoV-2 Virus like Particles produced by a single recombinant baculovirus generate potent neutralizing antibody that protects against variant challenge. bioRxiv. doi:10.1101/2021.11.29.470349. https://www.biorxiv.org/content/10.1101/2021.11.29.470349v1.full.
- Peer reviewed and published scientific report.
Sullivan, Edward, Po-Yu Sung, Weining Wu, Neil Berry, Sarah Kempster, Deborah Ferguson, Neil Almond, Ian M. Jones, and Polly Roy. 2022. “SARS-CoV-2 Virus-like Particles Produced by a Single Recombinant Baculovirus Generate Anti-S Antibody and Protect against Variant Challenge.” Viruses 14 (5): 914. https://doi.org/10.3390/v14050914. https://www.mdpi.com/1999-4915/14/5/914.