Patients with end-stage kidney disease (ESKD) on hemodialysis are at a higher risk of SARS-CoV-2 infection due to impaired immune responses and constant exposure to hospital facilities.
Previous reports show that a considerably large proportion (80-90%) of ESKD patients develop immunoglobulin G (IgG) antibodies against the spike protein receptor-binding domain (RBD) of SARS-CoV-2. However, these rates remain lower than those observed in healthy individuals. Dialysis patients have also been shown to attain lower antibody levels in response to mRNA vaccines as compared to healthy controls.
The data on long-term durability of humoral immune responses in hemodialysis patients following a standard two dose vaccine regimen is not available. Therefore, the team from the University of Virginia undertook this cohort study to characterize the time-dependent antibody decline in such patients, measuring monthly IgG antibody levels to the SARS-CoV-2 spike RBD for up to six months in fully vaccinated dialysis patients.
Study methods
Thirty-five hemodialytic patients who had received two doses of the BNT162b2 vaccine between January and February 2021 were enrolled for the current study. Blood samples were collected monthly starting from two months post-full immunization for up to six months.
Plasma was separated from the blood and tested for anti-SARS-CoV-2 antibodies against the spike S1 domain. Results were considered negative for BAU/mL of less than 25.6, borderline for BAU/mL greater than or equal to 25.6 but less than 35.2, and positive for BAU/mL greater than or equal to 35.2.
The team also performed qualitative detection of total antibodies to the SARS-CoV-2 nucleocapsid protein in order to confirm reported histories of prior infection and detect an undiagnosed prior infection.
A linear mixed model with a random slope and random intercept was used for evaluating temporal antibody levels accounting for patient-specific changes and variation over the entire follow-up period.
A multivariable model was used to estimate the slope of antibody levels by adjusting for selected patient characteristics including age, gender, prior SARS-CoV-2 infection, and immune suppression. In addition, based on the estimated intercepts and slopes for each subject from the unadjusted model, ten-month antibody levels were projected.
Antibody levels wane at a steady rate with a faster decay in older hemodialytic patients
Eighty-eight percent of patients were found qualitatively positive for IgG antibodies to spike protein, thus confirming the previous reports. The team observed a stable decline in IgG spike protein antibody levels from month to month, regardless of subgroup or initial antibody peak.
The mean baseline antibody level was 647.59 BAU/mL at two months; however, this declined to 491.4, 365.6, 302.0, and 177.9 BAU/mL at three, four, five, and six months following full vaccination, respectively. Taken together, mean antibody levels decayed by an average of 31% per month. Older patients experienced greater decay in the antibody levels at an additional 4% decline for a one-year increment in age.
This relatively stable decay rate suggests that peak attained antibody level post-vaccination is a predictive factor determining the duration of detectable IgG spike protein antibody levels.”
Immune suppression and prior infection impact post-vaccination antibody levels
The adjusted multivariable linear mixed model demonstrated that prior SARS-CoV-2 infection and immune suppression were significantly associated with the trajectory of antibody level, whereas age marginally impacted these levels. Patients with prior SARS-CoV-2 infection had five times higher antibody levels than infection naive patients and these remained higher six months post full vaccination at 888.01 BAU/mL in prior infected individuals versus 61.01 BAU/mL in infection naïve patients.
Immune suppressed patients, on average, had 65% lower antibody levels as compared to patients without immune suppression.
At six months post full immunization, 61% of patients maintained positive antibody levels, while 39% regressed to borderline or negative antibody levels. Surprisingly, patients already in the borderline or negative group are mostly drawn from the infection naive group, and not the immune-suppressed group.
The forecast of antibody levels predicted the regression of more than 65% of the study population to borderline or negative antibody status at ten-months post full vaccination. However, none of the previously infected patients were projected to lose detectable antibody levels at ten months.
These results suggest that dialysis patients vaccinated with BNT162b2 and without prior infection may benefit from receipt of a booster dose.”
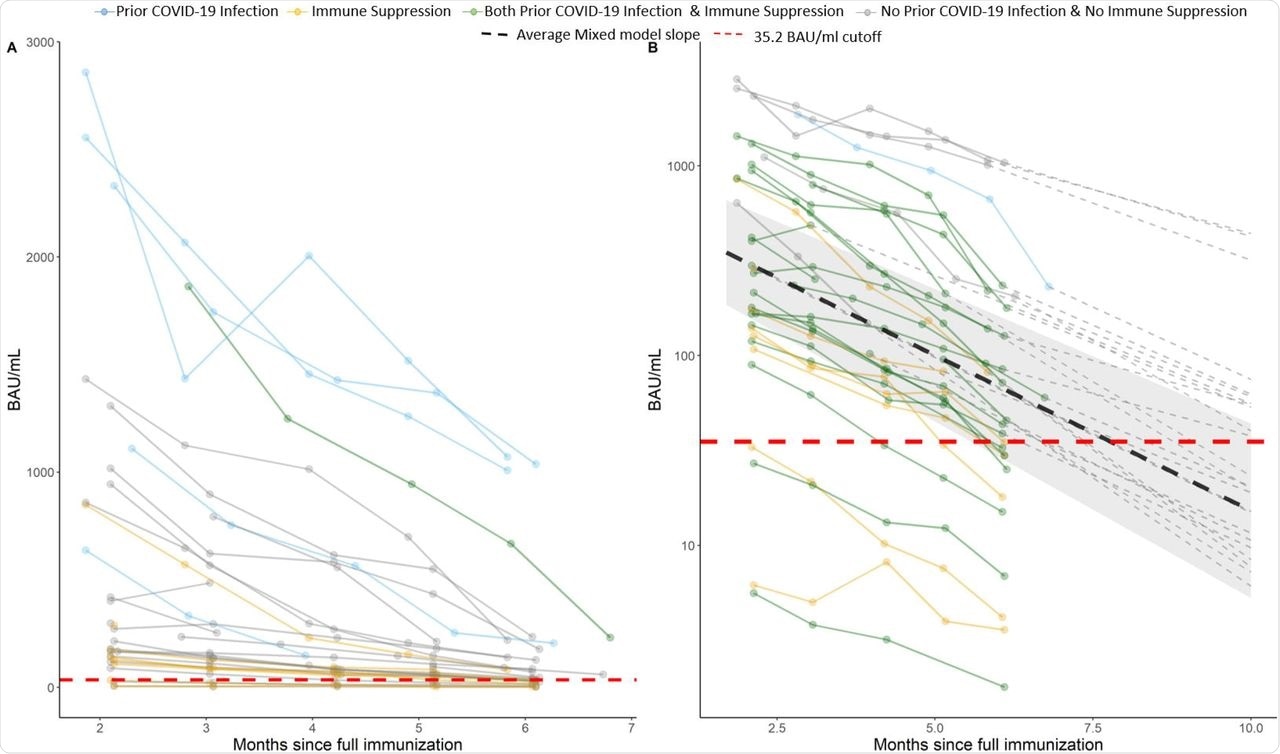 A) Observed antibody level of SARS-CoV-2, the lines are colored by prior COVID-19 infection and immune suppression status. B) Logarithmic scale (y axis) prediction graph on individuals post 10 month since full vaccination. The dark lines are observed values and the dotted lines are predicted values (dashed line not shown for subjects who are “negative” or “borderline” at month 6). Individual intercept and slope estimated from unadjusted linear mixed model were used for prediction. The cutoff for borderline/negative antibody level is 35.2 (red dashed line).
A) Observed antibody level of SARS-CoV-2, the lines are colored by prior COVID-19 infection and immune suppression status. B) Logarithmic scale (y axis) prediction graph on individuals post 10 month since full vaccination. The dark lines are observed values and the dotted lines are predicted values (dashed line not shown for subjects who are “negative” or “borderline” at month 6). Individual intercept and slope estimated from unadjusted linear mixed model were used for prediction. The cutoff for borderline/negative antibody level is 35.2 (red dashed line).
Limitations
The team warns to interpret the results with caution due to certain limitations of the study, including a small cohort sample size, a majority of the study population being African American, a large proportion of patients being immune-suppressed, and the results being derived from using the BNT162b2 vaccine only.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Goggins E., Sharma, B., Ma, J. Z., et al. (2021) Long term humoral immunity decline in hemodialysis patients following SARS-CoV-2 vaccination. medRxiv. doi:10.1101/2021.12.01.21265957, https://www.medrxiv.org/content/10.1101/2021.12.01.21265957v1.
- Peer reviewed and published scientific report.
Goggins, Eibhlin, Binu Sharma, Jennie Z. Ma, Jitendra Gautam, and Brendan Bowman. 2022. “Long-Term Humoral Immunity Decline in Hemodialysis Patients Following Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination: A Cohort Study.” Health Science Reports 5 (6). https://doi.org/10.1002/hsr2.854. https://onlinelibrary.wiley.com/doi/10.1002/hsr2.854.