In France, four coronavirus disease 2019 (COVID-19) vaccines, developed by Pfizer/BioNTech, Moderna, AstraZeneca, and Janssen-Cilag, have received emergency use authorization (EUA) and a rapid vaccination program had commenced to protect its citizens from the infection. The COVID-19 pandemic has been caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which has been characterized as highly transmissible, virulent, and has a high mortality rate.
In January 2021, elderly people and frontline workers, who were at a high risk of COVID-19 infection, were prioritized for SARS-CoV-2 vaccination. However, gradually, it was extended to younger age groups down to 12 years of age. Although the vaccination coverage rapidly increased, it slackened significantly by the end of the summer.
Study: Evaluating COVID-19 booster vaccination strategies in a partially vaccinated population: a modeling study. Image Credit: Studio Romantic/Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The need for booster vaccines
The emergence of SARS-CoV-2 variants occurred due to mutations. Scientists have categorized the variants as variants of concern (VOC) and variants of interest (VOI). In February 2021, SARS-CoV-2 Alpha VOC became dominant in metropolitan France, which was replaced by Delta VOC. At present, the Delta variant has become the dominant circulating strain and has been characterized as more virulent and transmissible than the original SARS-CoV-2 strain. It can also evade the immune response induced by vaccines or natural infection. Many studies have reported that the efficacy of the available COVID-19 vaccines has been lower in the case of the Delta strain compared to other SARS-CoV-2 variants. Previous studies have also shown waning of immune protection, elicited via vaccines or natural infection, with time. Owing to these reasons many countries have recommended COVID-19 booster shots for already vaccinated individuals.
France's government had initially recommended COVID-19 booster dose for people who are above 65years as well as other vulnerable groups, six months after completion of the two-dose vaccination regime. However, more recently, the eligibility for the booster dose has been extended to all adults.
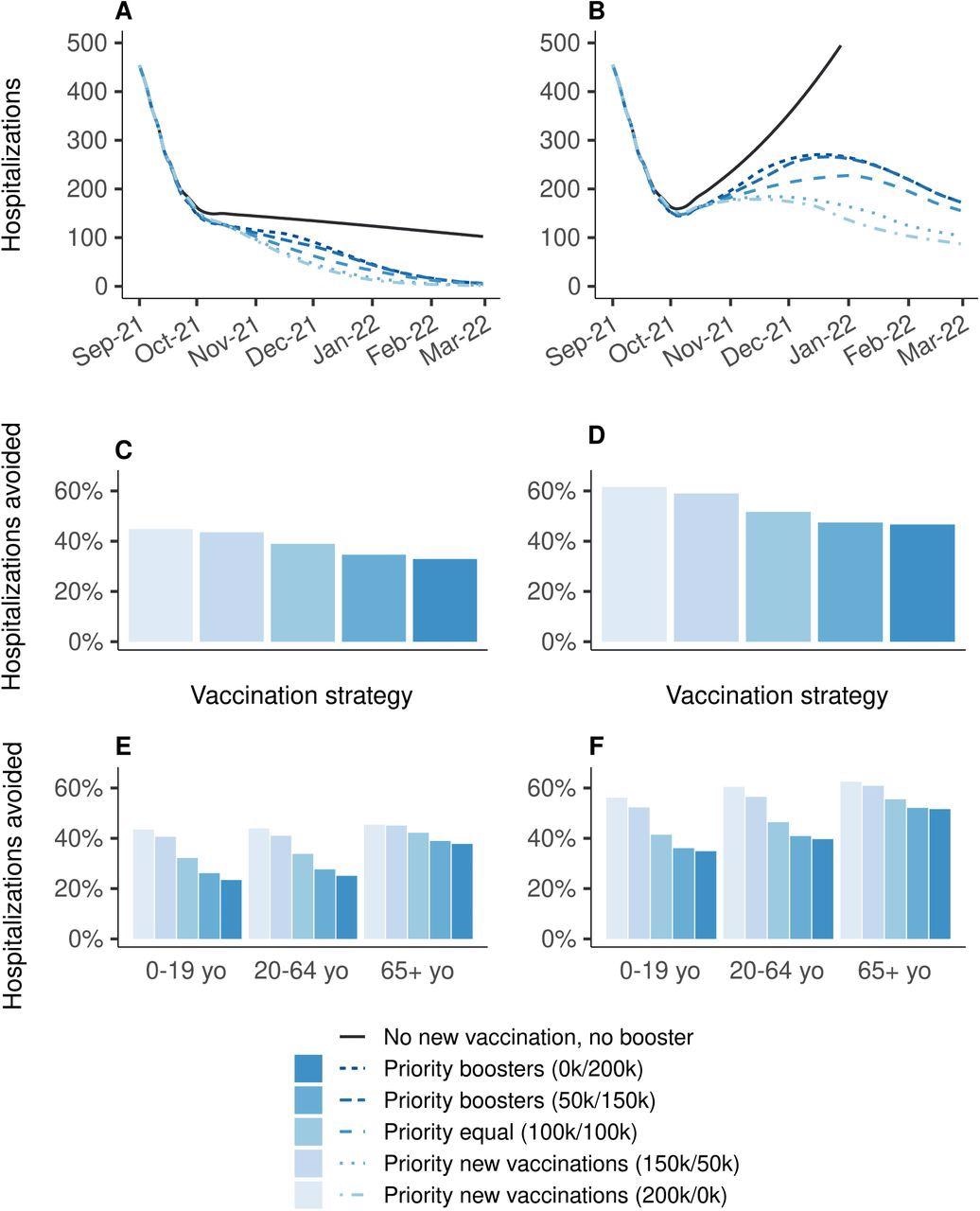 Effectiveness on hospitalizations of vaccination strategies varying in the allocation of 200,000 daily doses between primary vaccination and booster shots, over the period from September 1st, 2021 to March 1st, 2022, compared to a baseline scenario in which all vaccination is stopped on September 1st, 2021. (A, C, E) Vaccine efficacy decreased only for people aged 65 years and older. (B, D, F) Vaccine efficacy decreased for all age groups. (A,B) Daily new hospitalizations, (C, D) proportion of hospitalizations avoided, (E, F) proportion of hospitalizations avoided by age group. A prioritization strategy of (150k/50k) means 150k daily doses for primary vaccination and 50k daily booster doses until 90% coverage of one target population is reached, then all 200k daily doses are allocated to the other target population.
Effectiveness on hospitalizations of vaccination strategies varying in the allocation of 200,000 daily doses between primary vaccination and booster shots, over the period from September 1st, 2021 to March 1st, 2022, compared to a baseline scenario in which all vaccination is stopped on September 1st, 2021. (A, C, E) Vaccine efficacy decreased only for people aged 65 years and older. (B, D, F) Vaccine efficacy decreased for all age groups. (A,B) Daily new hospitalizations, (C, D) proportion of hospitalizations avoided, (E, F) proportion of hospitalizations avoided by age group. A prioritization strategy of (150k/50k) means 150k daily doses for primary vaccination and 50k daily booster doses until 90% coverage of one target population is reached, then all 200k daily doses are allocated to the other target population.
Effectiveness
In Europe, the number of COVID-19 cases has again started to increase. In this scenario, along with constraints in the global supply of vaccines, researchers believe it is extremely important to evaluate the effectiveness of booster vaccination campaigns. Researchers have used a deterministic, age-structured, compartmental model for this assessment. In this model, they fitted data that were associated with hospital admission and deaths. The authors validated sero-prevalence data in France to determine the effect of primary and booster vaccination strategies on morbidity and mortality.
This study is available on the medRxiv* preprint server. In this study, scientists made two assumptions, i.e., waning of immunity and enhanced virus transmissibility in winter.
Researchers compared vaccination strategies based on prioritization between primary vaccination and booster vaccination. This study revealed that the most beneficial strategy was to distribute all available doses of COVID-19 vaccines predominantly for primary vaccinations and the remaining doses for boosters. This result also emphasizes the fact that higher population immunity plays a greater part in protecting vulnerable groups than in protecting individuals.
The current study reported that in the scenario where if the effectiveness of vaccines were to be decreased among all age groups, the most effective approach to reduce hospitalization and death due to COVID-19 would be to provide booster vaccines for the 30-49 age group. In another scenario, where if the effectiveness of the vaccine were to be reduced for individuals aged 65 years or above, a different boosting vaccination could result in varying outcomes, in terms of, hospitalization and mortality. Therefore, this study suggested two mechanisms based on which healthcare policymakers could decide on which group to target for booster vaccination.
Researchers suggested that the decision must be made on the basis of immunization of vulnerable groups who are prone to severe infection and those with a high risk of transmission. The latter group will help reduce transmission of infection and, thereby, protect the population from further infection. Therefore, an optimal booster vaccination strategy would be based on protecting the vulnerable group as well as the population at large by inhibiting viral transmission. Researchers assumed that a recovered individual would not be reinfected again. Although some studies have reported on the breakthrough infection, not much evidence is available on the risk of re-infection after having recovered from COVID-19.
Conclusion
The model presented in this study helped determine the optimal vaccination strategy related to the primary and booster vaccination approach. Although this study has drawn a conclusion based on the data from metropolitan France, the authors believe that the observations would hold true for other countries as well. The emergence of the Omicron variant has indicated the importance of vaccination coverage throughout the world. Rapid global vaccination will help contain the COVID-19 pandemic sooner.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources