Mortality due to the coronavirus disease 2019 (COVID-19) is thought to be the result of respiratory failure in individuals who experience acute respiratory distress syndrome (ARDS) due to lung infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A new study published on the preprint server bioRxiv* demonstrates lethal infection of the olfactory epithelium in a mouse model that could help understand the mechanisms of disease and develop better preventive and therapeutic agents.
Background
The pathogenesis of COVID-19 has defied clear explanation, even two years into the pandemic, despite the use of advanced sequencing platforms and animal models. In most of these studies, the angiotensin-converting enzyme 2 (ACE2) receptor, which is the host cell receptor targeted by SARS-CoV-2, has been the focus.
SARS-CoV-2 is studded with spike glycoproteins that mediate attachment to the ACE-2 receptor and virus entry into the host cell. Cells and tissues that express this receptor at high levels are therefore thought to be susceptible to infection by this virus.
Manifestations of COVID-19 are explained in different ways, from direct cell infection to indirect tissue damage due to systemic inflammation. There remains a great need to identify the underlying mechanism by which COVID-19 causes cell and tissue injury by in vivo studies. These studies would likely uncover definite cellular targets and assist in the development of efficient antiviral agents.
Anosmia and respiratory difficulty are among the more characteristic features of acute COVID-19. Anosmia is the result of SARS-CoV-2 infecting the olfactory epithelium (OE) but is not considered to mediate lethal outcomes. However, hypoxemic respiratory failure is the leading cause of death in COVID-19.
Pneumonia that arises as a result of COVID-19 is considered to be due to direct lung infection or infection of lung macrophages. The terminal feature of COVID-19 is often rapidly progressive hypoxemia with pulmonary edema and ARDS. ARDS is not unique to COVID-19, as it commonly arises in cases of acute pancreatitis, sepsis, or other conditions associated with a cytokine storm.
The focus of the current study is to elucidate the mechanisms of respiratory failure in COVID-19 through the use of an engineered mouse model. The mouse ACE2 does not do so, which has led to the production of transgenic mice that express the human ACE2 (hACE2) that allow COVID-19 to be studied in these models.
The K18-hACE2 mouse model, with a predominantly epithelial expression of this receptor, has shown severe or lethal illness following infection with SARS-CoV-2. The authors of this study utilized a new mouse model that expresses hACE2 from the locus of the mouse ACE2 gene in order to enable its expression in the desired cells with the desired gain of function as a result.
Study findings
The investigators found that these mice expressed the hACE2 gene endogenously in the olfactory, intestinal, and renal epithelium, though there was no detectable expression in the lung in either mouse line. The researchers also found that they could prevent hACE2 expression by removing a crucial element in these mice.
Secondly, they found that the engineered mice developed a lethal infection when exposed to SARS-CoV-2 in 100% of animals by the sixth day. When hACE2 expression is prevented, the animals failed to become sick following exposure to a lethal dose of the virus.
Similar to the disease profile in humans, male mice became ill sooner than females, though both alike showed signs of lethal illness in all cases. Thus, the expression of hACE2 in an endogenous fashion led to the development of severe disease and respiratory distress, with a lethal outcome following SARS-CoV-2 exposure.
It has been assumed that Type 2 (AT2) lung epithelial cells are infected by SARS-CoV-2 as a result of its ACE2 expression, though at a low level, leading to acute lung injury (ALI) and ARDS. The investigators compared levels of the viral nucleocapsid (N) protein and ribonucleic acid (RNA) in the mouse lung of infected male animals.
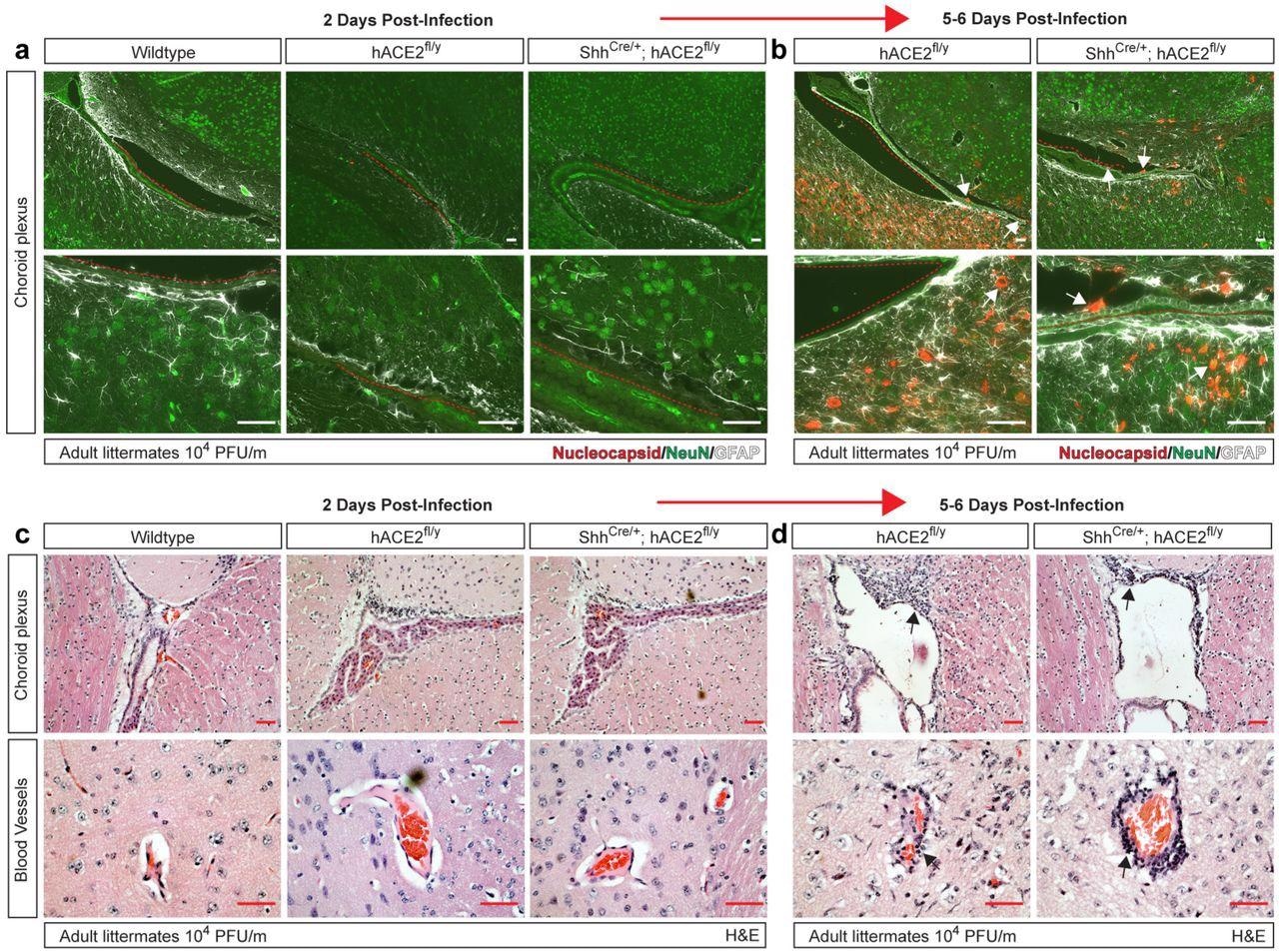 eningeal and vascular inflammation is associated with neuronal infection at the choroid plexus. a-b, Immunohistochemistry of SARS-CoV-2 nucleocapsid, neuronal NeuN and glial cell GFAP of the cerebral cortex adjacent to the choroid plexus 2 and 5-6 days after infection. Arrows indicated nucleocapsid staining. Red dotted lines trace ependymal cells of the choroid plexus. c-d, H&E staining of brain choroid plexus and cerebral cortex blood vessels 2 and 5-6 days after infection of hACE2fl/y and ShhCre/+; hACE2fl/y mice. Arrows indicate sites of immune cell infiltration. Representative of N=4 animals per genotype and timepoint. Scale bars 50 µm.
eningeal and vascular inflammation is associated with neuronal infection at the choroid plexus. a-b, Immunohistochemistry of SARS-CoV-2 nucleocapsid, neuronal NeuN and glial cell GFAP of the cerebral cortex adjacent to the choroid plexus 2 and 5-6 days after infection. Arrows indicated nucleocapsid staining. Red dotted lines trace ependymal cells of the choroid plexus. c-d, H&E staining of brain choroid plexus and cerebral cortex blood vessels 2 and 5-6 days after infection of hACE2fl/y and ShhCre/+; hACE2fl/y mice. Arrows indicate sites of immune cell infiltration. Representative of N=4 animals per genotype and timepoint. Scale bars 50 µm.
Interestingly, the lung epithelial cells in the hACE2-positive mice were found to have similar levels of viral RNA and N protein as compared to non-hACE2-expressing mice. In other words, non-AT2 cells also mediate SARS-CoV-2 infection of the lung.
Viral N protein, showing the presence of infection, was also found to be localized in alveolar type 1 (AT1) cells in the lungs of the infected hACE2-positive mice. Taken together, both AT1 and AT2 lung epithelial cells support SARS-CoV-2 infection through the ACE2 receptor. However, to date, ACE2 has not been detected in AT1 cells.
Thirdly, the investigators found that the presence of primary lung infection with SARS-CoV-2 was not required for severe disease and lethality in mice. Lethal disease set in following hACE2 ablation in the lung cells, despite a small delay in the onset of symptoms in those mice that lacked the receptor. By six days post-infection, both mouse models showed severe hypoxemic respiratory distress, with oxygen saturations resembling those in COVID-19 patients on mechanical ventilation.
Severe lung lesions similar to those in humans with lethal COVID-19 were observed in the new mouse model; however, these findings were not observed in wild-type mice following exposure to SARs-CoV-2. Evidence of systemic inflammation was observed in the raised levels of inflammatory proteins ICAM1 and Podoplanin (PDPN) in the alveolar epithelium of both mouse models, but not in the wild-type mice.
The lung capillary endothelium in the engineered mouse models also showed higher expression of the pro-coagulant protein von Willebrand’s Factor (vWF), which is also an inflammatory marker, thus indicating intravascular clot formation. These findings show that without primary SARS-CoV-2 infection of the lung epithelium, these mice still showed signs of acute lung injury (ALI) and hypoxemic respiratory failure, thereby indicating that inflammation and ARDS are mediators of respiratory failure and death in infected hACE2-expressing mice.
On the other hand, the respiratory epithelium (RE) and olfactory epithelium (OE) in the hACE2-positive mice showed high ACE2 expression and abundant viral nucleocapsid (N) protein at two days post-infection. At 5-6 days, N protein levels dropped significantly in these areas but were very high in the neighboring olfactory bulb, cerebral cortex, and hippocampus.
The N protein was present within the neurons and glial cells, with the reactive proliferation of the glial cells. The same reaction was seen, albeit at a lower level, in the brainstem, perhaps because it is farther from the OE. These findings suggest that the OE is infected but rapidly clears the virus in humans but this is followed by infection of the brain.
In human COVID-19, the presence of the virus in the choroid plexus within the cerebral ventricles has not been reported; however, an antiviral inflammatory response was observed. In the current study, mice also did not show the presence of the N protein in the choroid plexus or nearby neurons at two days post-infection; however, it was observed in a patchy distribution near the choroid plexus and the meninges at five days. At this time, immune cell infiltration was also observed along the lateral ventricle and the blood vessels of the cerebral cortex, which is suggestive of an inflammatory response.
These results raise the question of whether the brain can be infected by routes other than the OE. At present, available evidence suggests that SARS-CoV-2 first enters the OE and then the brain, probably through the olfactory sensory neurons (OSNs) that connect these two areas, are rich in ACE2 and show signs of infection early on the following exposure.
The researchers ruled out artefactual brain infection as a result of the abnormally high levels of hACE2 in the brain due to the genetic engineering by infecting another type of mouse line where the hACE2 protein was fused to a green fluorescent protein (GFP). In this model, brain ACE2 expression was undetectable, as in healthy wild-type mice and humans; however, viral N protein and reactive gliosis were both present following infection, with a few patches of N protein staining near the meninges. In addition, meningeal and perivascular inflammatory infiltrates were present.
“These studies confirm that neuronal infection takes place in animals that express levels of hACE2 in the brain like those in the wild-type mouse and human brain and provide additional evidence for a pathogenic link between infection of the OE, OSN and brain during lethal COVID-19.”
In contrast, the use of methimazole to ablate the OE while leaving the olfactory bulb and lung cells intact led to the prevention of weight loss and death for most of the hACE2-positive mice. There was no sign of brain infection either, similar to control mACE2-expressing mice. When hACE2 was expressed on the OE and cerebral cortex neurons, lethal infection with ALI occurred.
In another experiment, the researchers generated mice with ACE2 expression only in the lung but not in the forebrain or OE. This mouse line survived infection without severe disease or death, thus confirming that lung infection in hACE2-expressing mice is not adequate to cause weight loss and death.
Conversely, the infection of the OE and brain by SARS-CoV-2 is enough and essential to cause ALI, ARDS, and severe hypoxemic respiratory failure in severe COVID-19, as in the hACE2-expressing mice.
Implications
The current study confirms the key role of the ACE2 receptor in SARS-CoV-2 infection, but at often undetectable levels when assessed by standard methods. Secondly, lethal ARDS in COVID-19 may occur following infection outside the lungs.
In the individual patient, the pulmonary or extra-pulmonary infection may contribute to respiratory failure more dominantly depending on the presence of other illnesses and the vagaries of the immune response. However, OE infection is necessary for severe disease, probably beginning with early infection of sustentacular cells expressing the receptor.
SARS-CoV-2 spreads into the OSNs in close proximity within the first two days of infection, later propagating to other neurons that are farther away by the direct spread. This proposed mechanism fits observed findings, while also explaining the anosmia and other neurologic features of COVID-19 in humans.
The rapid movement of SARS-CoV-2 in an unpredictable distribution, coupled with the limited area and sample size of biopsies, may explain why some previous studies failed to find the virus in the brains of human non-survivors. In contrast, the whole brain and nasal cavity are examined for the virus, following a known exposure dose at a known time, thus allowing for highly reproducible detection of the virus.
“Our results demonstrate that acute lung injury can occur in the absence of pulmonary infection and identify the olfactory epithelium and cerebral neurons as critical cellular sites of infection during lethal COVID-19.”
These findings point to new targets for the development of therapeutic and preventive agents, such as drugs that prevent OE infection. These may include vaccines that induce mucosal immunity or drugs that prevent virus-ACE2 binding at the OE when inhaled or sprayed into the nasal cavity.
The mouse model itself deserves to be refined so as to yield more data on how acute SARS-CoV-2 infection produces chronic sequelae, thus allowing further detailed exploration of the pathogenetic mechanisms of human COVID-19.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.