Background
In the field of modern medicine, ribonucleic acids (RNAs) have gained significant attention for silencing genes (siRNA), expressing proteins like mRNA, or editing genes like the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system. Several chemical modifications of RNAs have been done to increase stability and reduce toxicity.
Recently, two mRNA-based coronavirus disease 2019 (COVID-19) vaccines developed by Pfizer/BioNTech and Moderna have shown high efficacy in preventing viral infection and symptomatic disease in both clinical trials and in the real world.
Lipid nanoparticle-based delivery systems have shown high efficacy in delivering RNA-based therapeutics and vaccines. The delivery efficacy of nanoparticles strictly depends on internalization, endosomal distribution, and RNA escape.
In the current study, scientists investigate the endosomal escape mechanism and cytotoxicity of six lipid nanoparticle-based mRNA formations with varied composition and efficacy in primary human adipocytes, fibroblasts, and HeLa cells. The composition of one formulation is similar to that which is used in the Moderna COVID-19 vaccine.
Internalization of lipid nanoparticle-based mRNA formations
The current study utilized the single-molecule fluorescence in situ hybridization (FISH) method to assess the internalization and endosomal distribution of tested formulations. The findings revealed that there is a difference in uptake and trafficking between lipid nanoparticles and mRNA.
Upon internalization, the formulation cargo is transported to either early endosomes for recycling or late endosomes for degradation. For effective and nontoxic delivery, lipid nanoparticles must transport mRNA to endosomal compartments, where they can be released into the cytosol without impairing endosomal functions.
The imaging of mRNA distribution in different compartments revealed that the distribution of the tested formulations in early endosomes is significantly higher than that in other compartments. The formulations with high-efficacy lipid nanoparticles exhibited higher distribution in early and recycling endosomes. However, no such distribution was observed in late endosomes, thereby indicating that this compartment is not significantly associated with mRNA delivery.
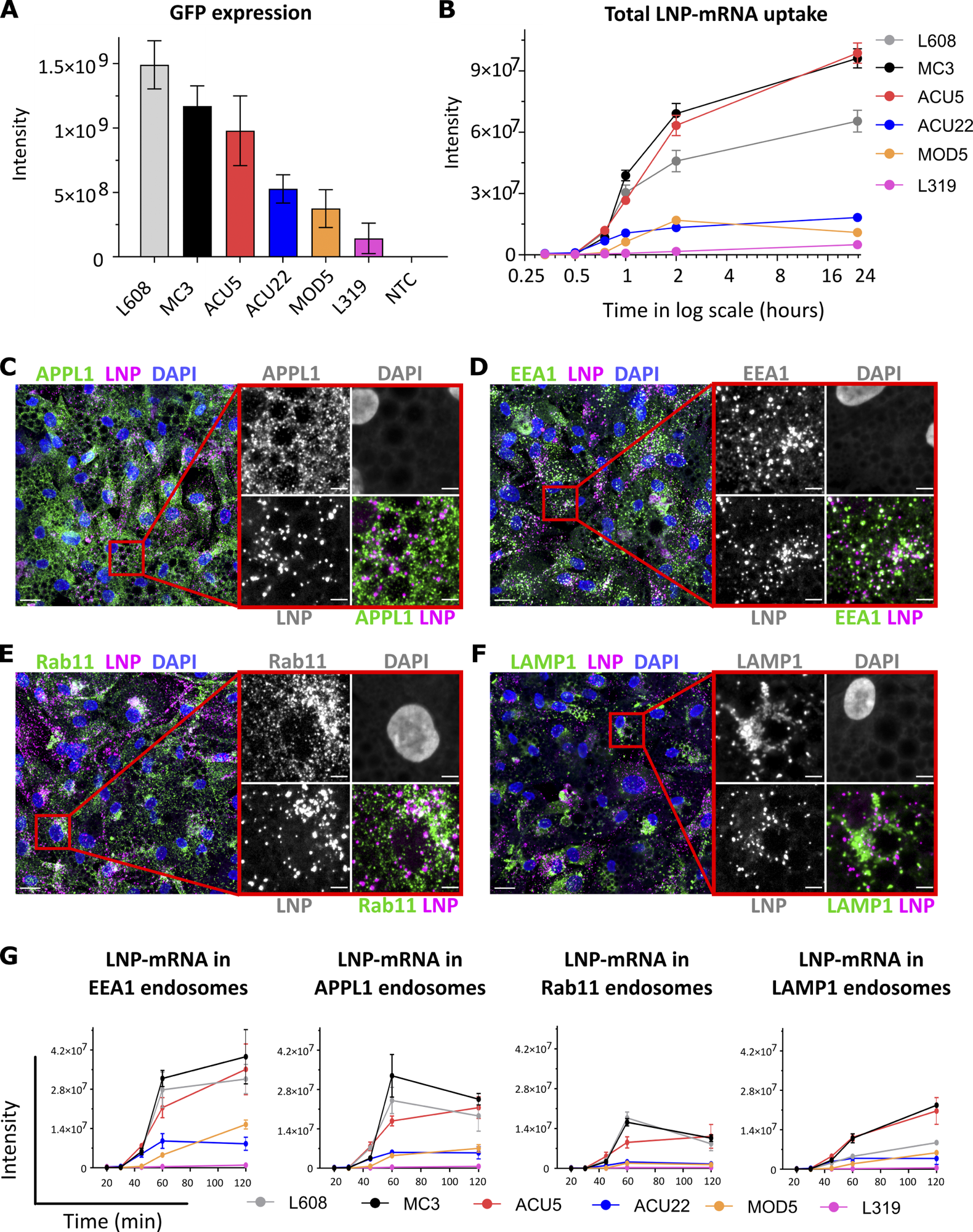 Comparative analysis of activity, endocytic uptake, and endosomal distribution of six LNP-mRNAs in primary human adipocytes. (A) Cells were incubated with various LNPs (1.25 ng/μl) formulated with eGFP-mRNA, fixed, and imaged after 24 h. The graph illustrates GFP expression for the indicated LNP-mRNA. n = 3 independent experiments (mean ± SEM). (B) Representative LNP-mRNA uptake kinetics curve. Cells incubated with LNP-mRNA as described above were fixed at the given time point, processed for smFISH to fluorescently label mRNA, and imaged by fluorescence microscopy. The quantification shows that LNP-mRNA uptake generally correlates to the GFP expression efficacy of LNP formulations displayed in A (e.g., MC3 versus L319). (C–F) Representative images of human primary adipocytes incubated with L608 LNP-mRNA for 2 h and immunostained with antibodies against endosomal markers (in green) as follows: APPL1 (C), EEA1 (D), Rab11 (E), and LAMP1 (F). Exogenous mRNA was detected by smFISH (labeled as LNP) and nuclei by DAPI. The magnified area is presented with split and merged color images. Scale bars are 20 μm in the overview and 5 μm in the magnified images. (G) Representative kinetics and endosomal distribution of the different LNP-mRNAs incubated with cells as described in C–F. n = 3 replicates (mean ± SEM).
Comparative analysis of activity, endocytic uptake, and endosomal distribution of six LNP-mRNAs in primary human adipocytes. (A) Cells were incubated with various LNPs (1.25 ng/μl) formulated with eGFP-mRNA, fixed, and imaged after 24 h. The graph illustrates GFP expression for the indicated LNP-mRNA. n = 3 independent experiments (mean ± SEM). (B) Representative LNP-mRNA uptake kinetics curve. Cells incubated with LNP-mRNA as described above were fixed at the given time point, processed for smFISH to fluorescently label mRNA, and imaged by fluorescence microscopy. The quantification shows that LNP-mRNA uptake generally correlates to the GFP expression efficacy of LNP formulations displayed in A (e.g., MC3 versus L319). (C–F) Representative images of human primary adipocytes incubated with L608 LNP-mRNA for 2 h and immunostained with antibodies against endosomal markers (in green) as follows: APPL1 (C), EEA1 (D), Rab11 (E), and LAMP1 (F). Exogenous mRNA was detected by smFISH (labeled as LNP) and nuclei by DAPI. The magnified area is presented with split and merged color images. Scale bars are 20 μm in the overview and 5 μm in the magnified images. (G) Representative kinetics and endosomal distribution of the different LNP-mRNAs incubated with cells as described in C–F. n = 3 replicates (mean ± SEM).
Distribution of mRNA in recycling endosomes
Mathematical modeling was applied to analyze the sequential transport of formulations from uptake to mRNA escape. The findings revealed that upon internalization, mRNA sequentially transverses early and recycling endosomal compartments and that the recycling compartment has the highest probability of mRNA escape.
Further analysis revealed that despite containing mRNA, large-sized early endosomes did not convert into late endosomes. This indicates inhibition of the progression of the internalized formulation.
Since impaired acidification of endosomes is the primary cause of inhibition of early endosome maturation, the scientists measured the pH of different endosomal compartments after prolonged uptake of the formulation. The findings revealed that the majority of formulation-containing endosomes have higher pH values that are not optimal for the maturation of early endosomes to late endosomes.
Furthermore, the findings revealed that endosomes containing large amounts of lipid nanoparticles have more severe impairment in acidification than those containing a lesser amount. However, despite not severely impairing acidification, endosomes with a low accumulation of lipid nanoparticles significantly inhibited the degradation of the formulation.
Taken together, these observations indicate that prolonged uptake of formulations causes impaired endosomal acidification, which subsequently leads to the accumulation of mRNA in compartments that are not associated with effective delivery.
Multicolor single-molecule localization microscopy was used to locate mRNA packed in single lipid nanoparticles, as well as unpacked mRNAs in sub-endosomal compartments. The lipid reorganization in acidic pH facilitated the unpacking and release of mRNA from lipid nanoparticles into the endosomal lumen and subsequent escape of mRNA from the endosomal lumen into the cytoplasm.
Further analysis revealed that the escape of mRNA occurred primarily from small-sized early endosomes and recycling tubular endosomes.
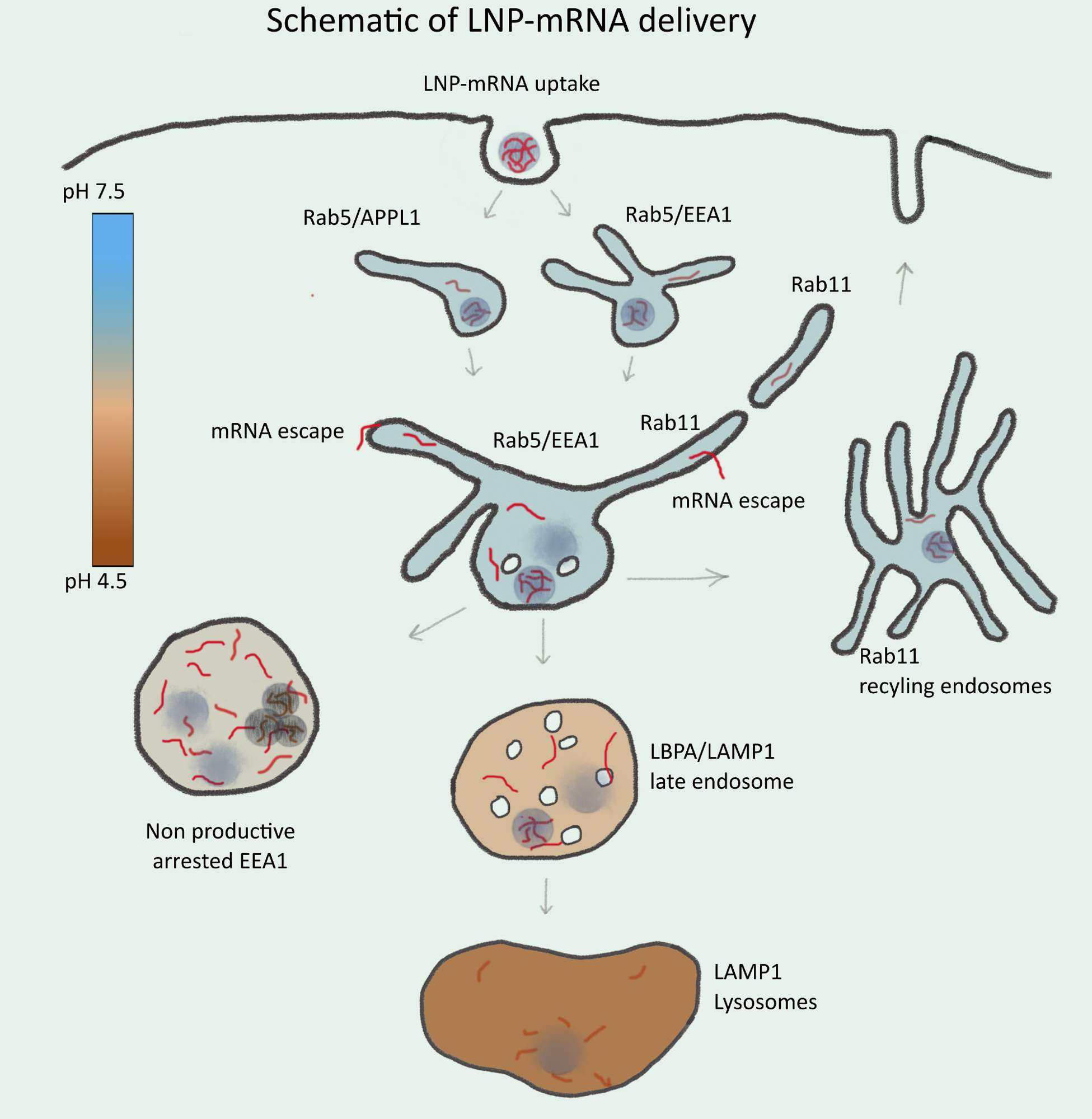 Schematic illustration of endosomal-mediated LNP-mRNA delivery. LNP-mRNAs are taken up in cells via endocytosis and sequentially transported to various endosomal compartments. Under normal conditions, these endosomal compartments maintain a characteristic pH (see heat map) for their functionality. Following uptake, mRNA in endosomes can be detected as individual LNP-mRNAs similar to the starting material by SMLM. Acidification of endosomal lumen leads to release of mRNA from LNP and escape from the endosomal lumen into the cytoplasm. Escape occurs mainly from small APPL1+, EEA1+, and/or Rab11+ tubular endosomes. Over time, the majority of LNP-mRNA accumulates in large, EEA1 endosomes where individual compact LNPs are disrupted, and mRNA signal becomes amorphous. Large endosomes become deficient in acidification, maturation arrested, and nonproductive for delivery. This may account for cytotoxicity of LNPs. Late endosomes and lysosomes are not favorable for mRNA escape.
Schematic illustration of endosomal-mediated LNP-mRNA delivery. LNP-mRNAs are taken up in cells via endocytosis and sequentially transported to various endosomal compartments. Under normal conditions, these endosomal compartments maintain a characteristic pH (see heat map) for their functionality. Following uptake, mRNA in endosomes can be detected as individual LNP-mRNAs similar to the starting material by SMLM. Acidification of endosomal lumen leads to release of mRNA from LNP and escape from the endosomal lumen into the cytoplasm. Escape occurs mainly from small APPL1+, EEA1+, and/or Rab11+ tubular endosomes. Over time, the majority of LNP-mRNA accumulates in large, EEA1 endosomes where individual compact LNPs are disrupted, and mRNA signal becomes amorphous. Large endosomes become deficient in acidification, maturation arrested, and nonproductive for delivery. This may account for cytotoxicity of LNPs. Late endosomes and lysosomes are not favorable for mRNA escape.
Study significance
The current study describes the sequential endosomal transport process of lipid nanoparticle-based mRNA formulations that lead to the effective delivery of mRNA into the cytoplasm. The findings presented here will help guide the development of new mRNA-based formulations with high efficacy and low-toxic delivery.
For effective delivery of mRNA, the best approach would be the development of lipid nanoparticles that can be distributed evenly between endosomal compartments or preferentially sorted to recycling tubules.
Furthermore, this study indicates that gradual accumulation of lipid nanoparticles in large endosomes leads to impaired acidification, inhibition of maturation, and disrupted mRNA delivery. These cytotoxic effects of lipid nanoparticles can be avoided by effective compartmentalization of mRNA-based formulations.
Journal reference:
- Paramasivam, P., Franke, C., Stoter, M., et al. (2021). Endosomal escape of delivered mRNA from endosomal recycling tubules visualized at the nanoscale. Journal of Cell Biology. doi:10.1083/jcb.202110137.