
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The emergence of more infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants, combined with the slow and inequitable global vaccination distribution, highlights a pressing need for optimal vaccination globally to achieve rapid population-level immunity.
Several studies have shown that prior infection bolsters the immune response in fully vaccinated individuals. However, more research is required to determine the durability of the antibody response after a single vaccine dose in individuals with prior SARS-CoV-2 infection.
About the study
Researchers in the U.K. recently investigated whether a single vaccine dose in previously infected individuals could elicit a similar initial and subsequent antibody response as fully vaccinated individuals without previous infection.
The three vaccines included in the current study were the Pfizer BTN162b2, Moderna mRNA-1273, and AstraZeneca ChAdOx1. The researchers studied these vaccines for their ability to induce anti-spike immunoglobulin G (IgG) levels after a single vaccination. The candidates with at least one antibody measurement 91 days before the first vaccination were evaluated until the second vaccination or a breakthrough infection after a single dose.
Of the 80,611 participants who received ChAdOx1 between December 8, 2020, and October 18, 2021, 12.6% had a prior infection. Comparatively, 17.1% of the 56,024 participants who received BNT162b2 had a prior infection before the first dose, whereas of the 3,545 participants who received mRNA-1273, 779 (22%) were previously infected.
More than 53% of the participants were females, of which 1.7% were healthcare workers, and 24.3% reported having chronic health conditions. The participants who did not have anti-spike antibodies to the first vaccine dose were excluded from the study.
“If a single vaccination invokes effective protection among those with prior infection, changing vaccine prioritization as an interim measure may deliver higher population-level immunity faster, and may also make vaccination programs more affordable.”
Study findings
The team found a median peak anti-spike immunoglobulin G (IgG) level of 549 ng/ml and a median half-life of 165 days in those with prior infection who received ChAdOx1 in the reference category that comprised people who were 50 years or more, females, ethnically white, and those without long-term health conditions. This was higher than that of fully vaccinated participants without prior infection, who showed a median peak of 271 ng/ml and a median half-life of 85 days. The participants without previous infection had lower peak levels of about 150 ng/ml and shorter half-lives of 105 days after a single dose for the same reference category.
In those who received the BNT162b2 vaccine and had a history of COVID-19, the median peak antibody level was 537 ng/ml and half-life was 236 days in the reference category. This peak level was lower than that of those who received two doses of BNT162b2 without prior infection, who showed a median peak of 793 ng/ml. In addition, the half-life in the fully vaccinated group was shorter at 111 days.
The median peak antibody level and half-life were 605 ng/ml and 572 days, respectively, for participants who received a single dose of mRNA-1273 with the previous infection, while these levels were 501 ng/ml and 71 days, respectively, for previously infected participants after their first dose for the reference category. No data was available for participants who received two doses of mRNA-1273.
The anti-spike IgG levels were higher for all three vaccines in participants with prior infection after their first dose compared to those who received two doses without previous infection. Prior infection significantly bolstered antibody levels, whereas the seropositivity rate was higher in females and those without long-term health conditions for all three vaccines.
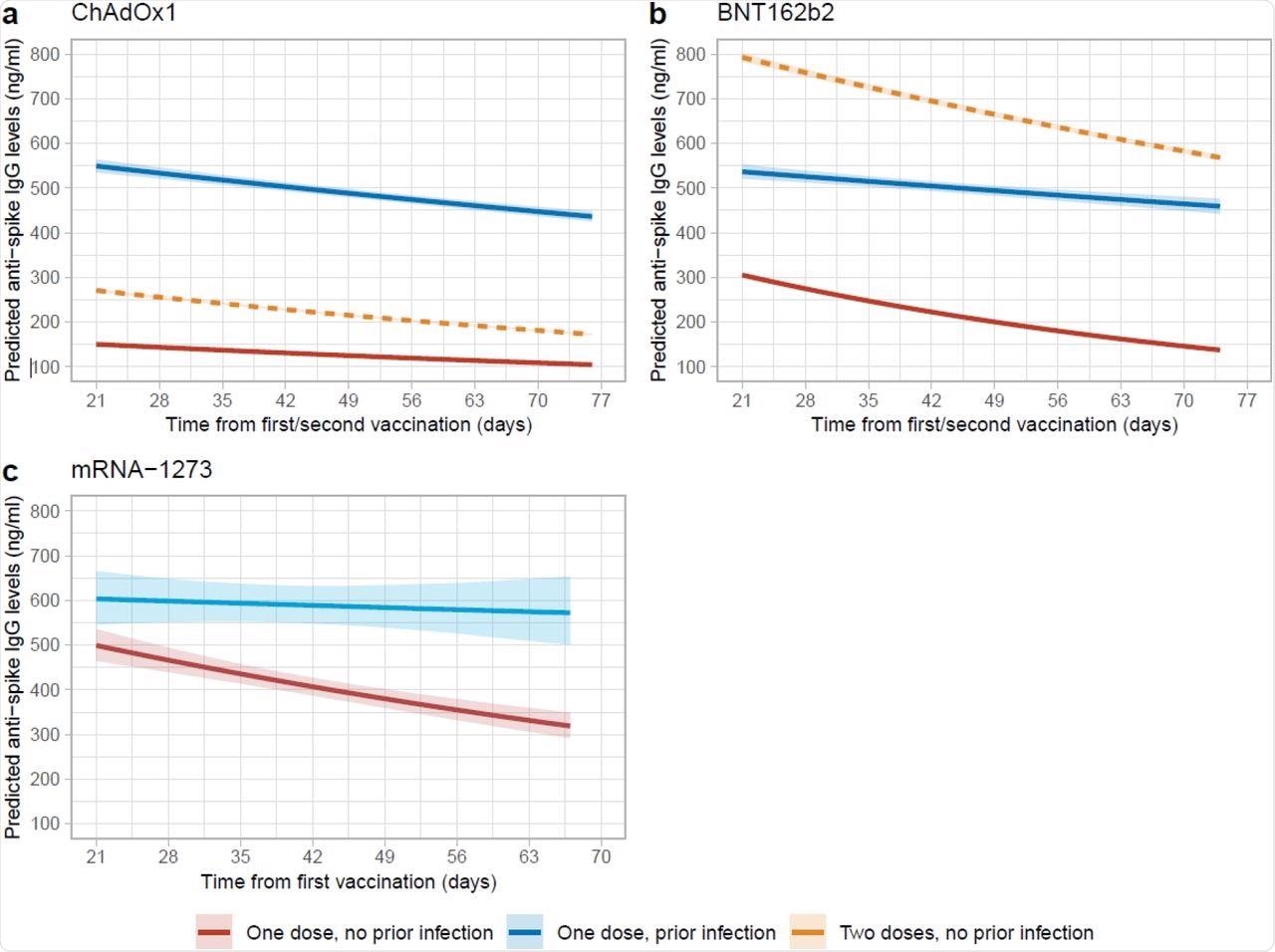 Posterior predicted trajectories of anti-spike IgG levels from 21 days post-first dose by prior infection status. a, 62,142 participants who received at least a single ChAdOx1 vaccination. b, 36,064 participants who received at least a single BNT162b2 vaccination. c, 2,688 participants who received at least a single mRNA-1273 vaccination. Plotted at reference categories: 50 years, female, white ethnicity, not reporting a long-term health condition, not a healthcare worker, and deprivation percentile=60. Peak levels for participants ≥60 years with single ChAdOx1 or BNT162b2 vaccination were at 28 days. Orange dotted lines in panel a and b were predicted trajectories post-second dose for 92,584 and 51,034 participants who received two ChAdOx1 and BNT162b2 vaccination without prior infection reproduced from our previous analysis, plotted at the same reference categories.
Posterior predicted trajectories of anti-spike IgG levels from 21 days post-first dose by prior infection status. a, 62,142 participants who received at least a single ChAdOx1 vaccination. b, 36,064 participants who received at least a single BNT162b2 vaccination. c, 2,688 participants who received at least a single mRNA-1273 vaccination. Plotted at reference categories: 50 years, female, white ethnicity, not reporting a long-term health condition, not a healthcare worker, and deprivation percentile=60. Peak levels for participants ≥60 years with single ChAdOx1 or BNT162b2 vaccination were at 28 days. Orange dotted lines in panel a and b were predicted trajectories post-second dose for 92,584 and 51,034 participants who received two ChAdOx1 and BNT162b2 vaccination without prior infection reproduced from our previous analysis, plotted at the same reference categories.
Conclusions
The study results showed that a single COVID-19 vaccine dose in previously infected individuals can significantly boost their immune response to SARS-CoV-2. Prior infection produced higher antibody levels and longer half-lives after vaccination that are comparable to those without previous infection after two vaccinations.
The antibody levels were higher by 100-400 ng/ml and the subsequent reduction of antibodies was also slower by 60-500 days in those with prior infection. Further, these individuals can remain protected for at least one year after a single dose of any of the three vaccines studied.
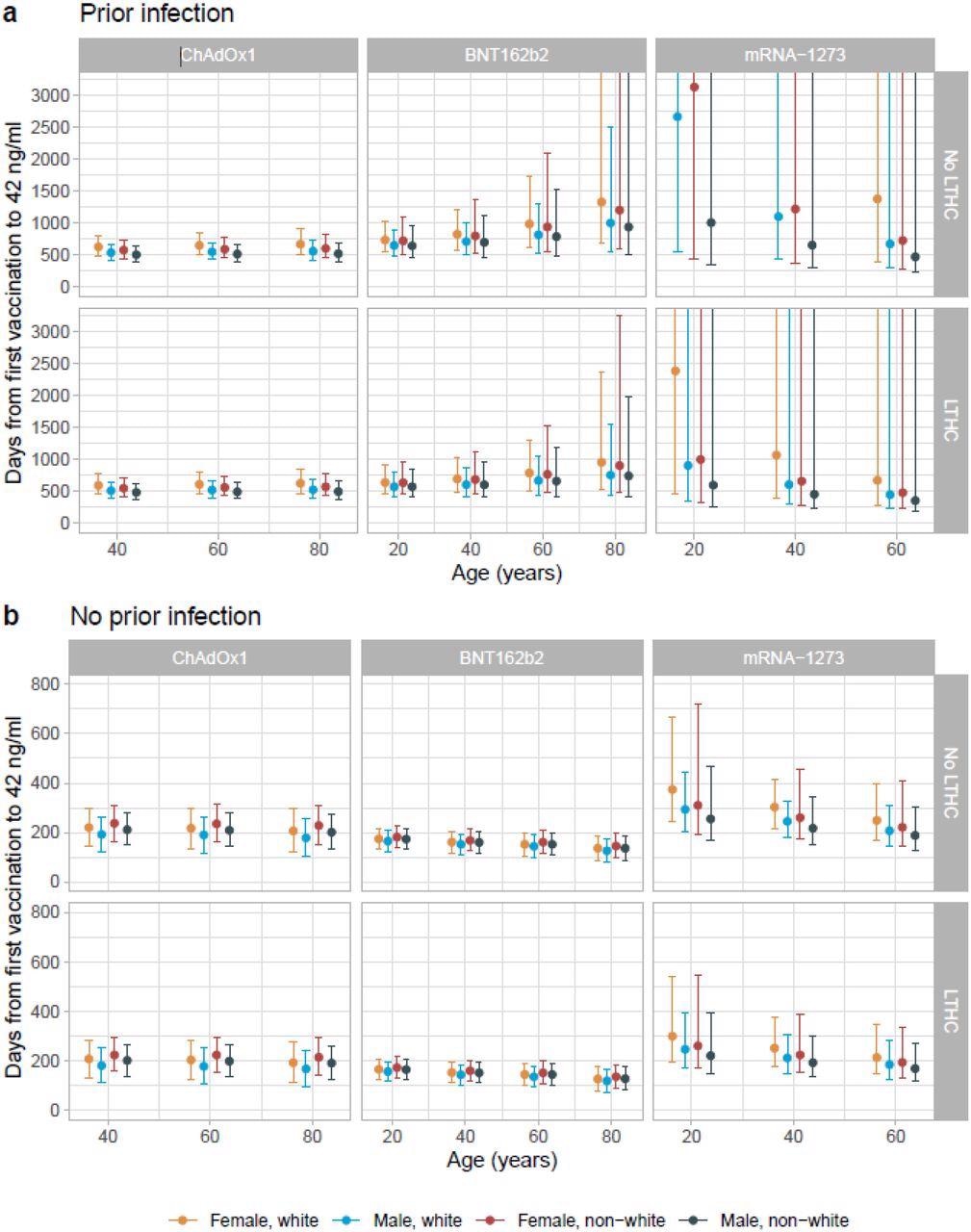 Posterior predicted days (95% credible interval) from the first vaccination to the positivity threshold of 42 ng/ml in those with evidence of prior infection (panel a) and without evidence of prior infection (panel b). Estimates were separated by age, sex, ethnicity, long-term health condition (LTHC), and vaccine type. The y-axis is truncated at 3,000 days (panel a) for visualization. For ChAdOx1, the 20-year-old group is not plotted because the vast majority of those receiving ChAdOx1 were ≥40 years. For mRNA-1273, the 80-year-old is not plotted because the vast majority of those receiving mRNA-1273 were ≤60 years. In panel a, 20 and 40-year-old white female without LTHC is not plotted in mRNA-1273 because their antibody levels were not estimated to decline so no duration could be estimated. Equivalent estimates after the second vaccination are provided in
Posterior predicted days (95% credible interval) from the first vaccination to the positivity threshold of 42 ng/ml in those with evidence of prior infection (panel a) and without evidence of prior infection (panel b). Estimates were separated by age, sex, ethnicity, long-term health condition (LTHC), and vaccine type. The y-axis is truncated at 3,000 days (panel a) for visualization. For ChAdOx1, the 20-year-old group is not plotted because the vast majority of those receiving ChAdOx1 were ≥40 years. For mRNA-1273, the 80-year-old is not plotted because the vast majority of those receiving mRNA-1273 were ≤60 years. In panel a, 20 and 40-year-old white female without LTHC is not plotted in mRNA-1273 because their antibody levels were not estimated to decline so no duration could be estimated. Equivalent estimates after the second vaccination are provided in
Single-dose vaccination aimed at those previously infected can protect populations with poor access to vaccines as an interim measure until global vaccine distribution capacity is improved. The current study offers evidence-based immune response data that could help global bodies amend and optimize their current vaccination strategies so that population-level immunity is achieved rapidly and effectively.
This is particularly important at a time when the incidence of SARS-CoV-2 cases is increasing and vaccine access is limited in many countries. The optimization of vaccination strategies can therefore minimize the incidence of new cases, deaths, morbidity, and socioeconomic damage caused by the COVID-19 pandemic.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Wei, J., Matthews, P. C., Stoesser, N., et al. (2021). The challenge of limited vaccine supplies: impact of prior infection on anti-spike IgG antibody trajectories after a single COVID-19 vaccination. medRxiv. doi:10.1101/2021.12.08.21267353. https://www.medrxiv.org/content/10.1101/2021.12.08.21267353v1.
- Peer reviewed and published scientific report.
Wei, Jia, Philippa C. Matthews, Nicole Stoesser, Ian Diamond, Ruth Studley, Emma Rourke, Duncan Cook, et al. 2022. “SARS-CoV-2 Antibody Trajectories after a Single COVID-19 Vaccination with and without Prior Infection.” Nature Communications 13 (1). https://doi.org/10.1038/s41467-022-31495-x. https://www.nature.com/articles/s41467-022-31495-x.