The Coronavirus disease 2019 (COVID-19) pandemic, which has been caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a global health emergency. Therefore, understanding the mechanisms and impact of booster vaccinations could immensely facilitate decisions concerning vaccination programs.
 Study: A third vaccination with a single T cell epitope protects against SARS-CoV-2 infection in the absence of neutralizing antibodies. Image Credit: Meletios Verras / Shutterstock
Study: A third vaccination with a single T cell epitope protects against SARS-CoV-2 infection in the absence of neutralizing antibodies. Image Credit: Meletios Verras / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Vaccines elicit neutralizing antibodies against the spike protein of SARS-CoV-2, but the antibodies decline over time. Furthermore, SARS-CoV-2 mutation rates are high, and certain mutations in its spike protein can evade vaccine-induced immune responses. Some high-risk groups, including transplant recipients, auto-immune disease patients who are on selected immunosuppressive regimens, etc., have lower humoral and cellular immunity after vaccination. Increasingly, third vaccinations are being given to solid organ transplant recipients as standard care.
Booster vaccinations have so far shown promising results, but concerns remain regarding the risk groups mentioned above. In addition, these booster vaccinations may result in enhanced T cell responses, which should contribute to the control of SARS-CoV-2. Previous research has shown that T cells can mediate protection by themselves against SARS-CoV-1, but their efficacy against SARS-CoV-2 is unclear. Therefore, in the current study, researchers investigated the capacity of single B cell and T cell epitope-containing peptide vaccines to elicit protection against SARS-CoV-2 infection. To this end, they used the K18-hACE2 transgenic mouse model.
Main Findings
The study revealed that only a third vaccination with a long peptide harboring a single T cell epitope provided full protection. The authors claimed that this is the first study to demonstrate that vaccine-elicited CD8+ T-cells can protect against SARS-CoV-2 without the help of virus-specific CD4+ helper T cells or neutralizing antibodies. They, however, stressed the administration of the vaccine in a booster setting requiring at least two boosters.
These results are highly relevant in the light of current deliberations on a third vaccine, as the current vaccines elicit virus-specific T cells. Besides, the results could also guide the development of T-cell-focused vaccines. The latter would greatly benefit risk groups, such as patients with leukemia, autoimmune diseases, etc., who have impaired antibody responses and may rely on T cell-eliciting vaccines for protection.
An important fact to note is that the DNA vaccine platform used was highly efficient in generating neutralizing antibodies. As a result, it provided protection against SARS-CoV-2 infection. The results highlighted the inferiority of the antibody responses to single linear B cell epitopes. However, it was not possible to exclude the possibility that single linear B cell epitopes might exist that could elicit neutralizing antibodies. In scenarios where antibodies mediate protection by other mechanisms, the B cell-SLP platform may be quite valuable. Scientists stated that the addition of CpG and IFA as adjuvants and the insertion of a CD4+ T helper cell epitope to the linear B cell epitope vaccine was found to be extremely important to derive antibody responses.
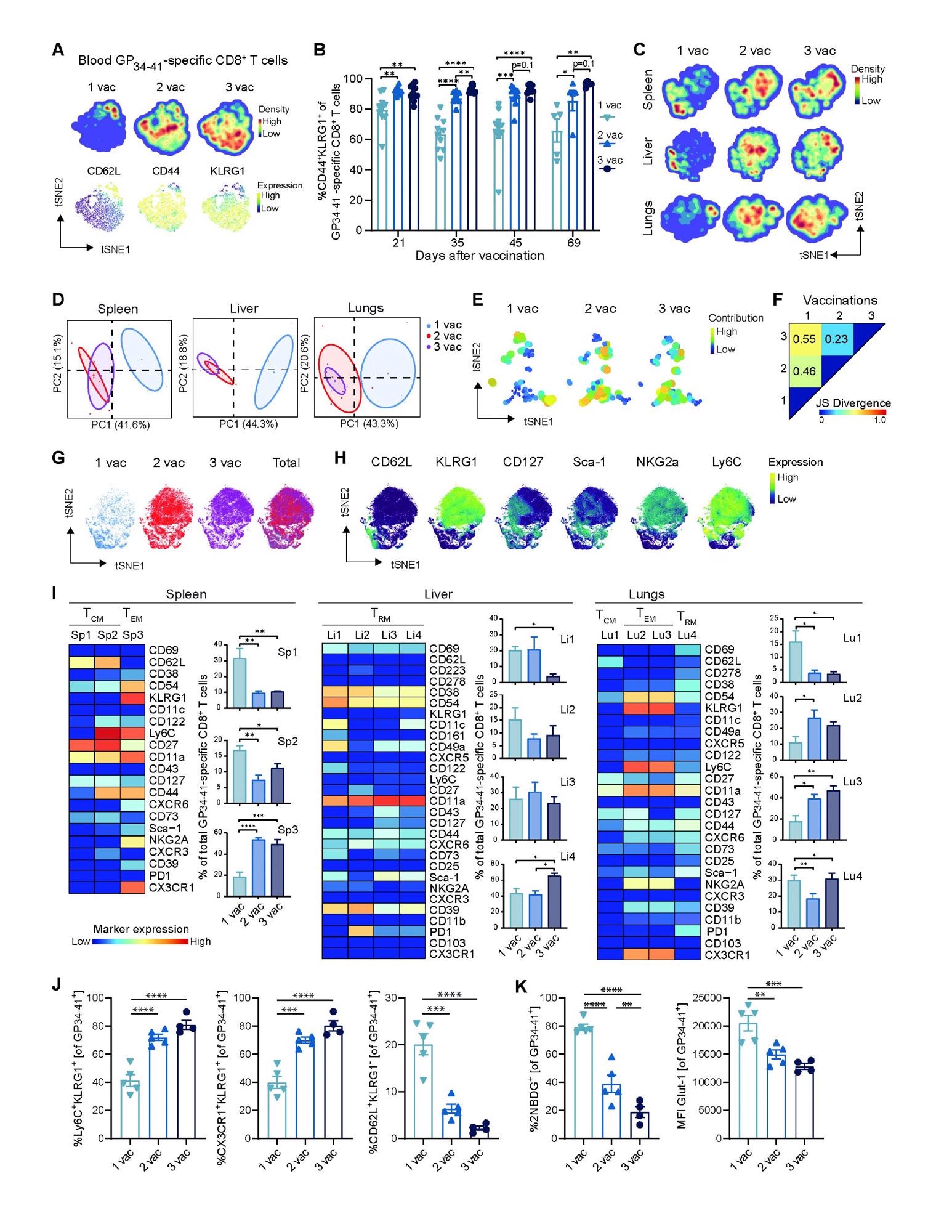 Boosting impact on the phenotype of vaccine-specific CD8+ T cells. (A) tSNE maps describing the local probability density of GP34-41-specific CD8+ T cells stained for CD62L, CD44, KLRG1 at day 71 after 1, 2 and 3 vaccinations. (B) Frequencies of CD44+ KLRG1+ GP34-41-specific CD8+ T cells in blood. Bar graphs represent means ± SEM. Symbols represent individual mice. (C) tSNE maps describing the local probability density of GP34-41-specific CD8+ T cells in spleen, liver and lungs stained at day 71 after 1, 2 and 3 vaccinations with the mass cytometry panel. (D) Principal Component Analysis illustrating the phenotypic dissimilarity of GP34-41-specific CD8+ T cells per tissue upon multiple vaccinations. (E) tSNE maps showing GP34-41-specific CD8+ T cell clusters per vaccination. Clusters with similar composition profiles across samples end up close together in the map. The varying dot size and color in this cluster tSNE map shows the average cluster normalized frequencies per vaccination/tissue group. (F) Pairwise Jensen-Shannon Divergence plots of the tSNE map obtained from all samples of GP34- 41-specific CD8+ T cells grouped by vaccinations. (G) tSNE embedding of GP34-41-specific CD8+ T cells isolated from vaccinated mice and multiple tissues in one analysis. Cells are color-coded per vaccination. (H) The expression intensity of the cell-surface markers on the GP34-41-specific CD8+ T cells. The color of the cells indicates ArcSinh5-transformed expression values for a given marker analyzed. (I) Heat maps of GP34-41- specific CD8+ T cell clusters in the spleen, liver and lungs of mice that received multiple vaccinations. Clusters were selected based on their significant difference and categorized in TCM, TEM and TRM subsets. Bar graphs indicate the average percentage (+ SEM) of each CD8+ T cell cluster within the GP34-41-specific CD8+ T-cell population elicited by one, two and three vaccinations. (J) Cell surface marker (Ly6C+ KLRG1+ , CX3CR1+ KLRG1+ and CD62L+ KLRG1- ) expression on GP34-41-specific CD8+ T cells at day 66 in spleen after 1, 2 or 3 vaccinations. Bar graphs represent means ± SEM. Symbols represent individual mice. Representative data of two independent experiments. (K) Glucose analog 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)- 2-deoxyglucose (2-NBDG) uptake and GLUT1 expression of splenic GP34-41-specific CD8+ T cells at day 66 in after 1, 2 and 3 vaccinations. Symbols represent individual mice. Bar graphs represent means ± SEM. Representative data of two independent experiments. A one-way ANOVA test with repeated measurements was used for statistical analysis. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Boosting impact on the phenotype of vaccine-specific CD8+ T cells. (A) tSNE maps describing the local probability density of GP34-41-specific CD8+ T cells stained for CD62L, CD44, KLRG1 at day 71 after 1, 2 and 3 vaccinations. (B) Frequencies of CD44+ KLRG1+ GP34-41-specific CD8+ T cells in blood. Bar graphs represent means ± SEM. Symbols represent individual mice. (C) tSNE maps describing the local probability density of GP34-41-specific CD8+ T cells in spleen, liver and lungs stained at day 71 after 1, 2 and 3 vaccinations with the mass cytometry panel. (D) Principal Component Analysis illustrating the phenotypic dissimilarity of GP34-41-specific CD8+ T cells per tissue upon multiple vaccinations. (E) tSNE maps showing GP34-41-specific CD8+ T cell clusters per vaccination. Clusters with similar composition profiles across samples end up close together in the map. The varying dot size and color in this cluster tSNE map shows the average cluster normalized frequencies per vaccination/tissue group. (F) Pairwise Jensen-Shannon Divergence plots of the tSNE map obtained from all samples of GP34- 41-specific CD8+ T cells grouped by vaccinations. (G) tSNE embedding of GP34-41-specific CD8+ T cells isolated from vaccinated mice and multiple tissues in one analysis. Cells are color-coded per vaccination. (H) The expression intensity of the cell-surface markers on the GP34-41-specific CD8+ T cells. The color of the cells indicates ArcSinh5-transformed expression values for a given marker analyzed. (I) Heat maps of GP34-41- specific CD8+ T cell clusters in the spleen, liver and lungs of mice that received multiple vaccinations. Clusters were selected based on their significant difference and categorized in TCM, TEM and TRM subsets. Bar graphs indicate the average percentage (+ SEM) of each CD8+ T cell cluster within the GP34-41-specific CD8+ T-cell population elicited by one, two and three vaccinations. (J) Cell surface marker (Ly6C+ KLRG1+ , CX3CR1+ KLRG1+ and CD62L+ KLRG1- ) expression on GP34-41-specific CD8+ T cells at day 66 in spleen after 1, 2 or 3 vaccinations. Bar graphs represent means ± SEM. Symbols represent individual mice. Representative data of two independent experiments. (K) Glucose analog 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)- 2-deoxyglucose (2-NBDG) uptake and GLUT1 expression of splenic GP34-41-specific CD8+ T cells at day 66 in after 1, 2 and 3 vaccinations. Symbols represent individual mice. Bar graphs represent means ± SEM. Representative data of two independent experiments. A one-way ANOVA test with repeated measurements was used for statistical analysis. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
More in-depth studies revealed that a third vaccination not only resulted in the superior generation of CD8+ TEM cells in circulation but also of CD8+ TRM cells in the liver and lungs. This result aligns with another recent human study, which showed that a third vaccination in kidney transplant recipients led to increased circulating polyfunctional CD4+ T cells. Future research could analyze whether a third dose of the mRNA vaccine in healthy individuals is also associated with increased T cell immunity.
Scientists highlighted that the increase of TEM and TRM cells in the lungs post the third vaccination might be critical as the lungs are the primary entry point for SARS-CoV-2. Moreover, the efficient formation of the TRM cells in the liver, mainly observed after the third vaccination, could contribute to protection as these cells have superior potential to differentiate into ex-TRM cells.
Conclusion
The current study showed that a third vaccination with a synthetic vaccine containing a single CD8+ T cell epitope results in protection against SARS-CoV-2, which could be attributed to an improved quantitative and qualitative CD8+ T cell response after the third vaccination. This is highlighted by greater numbers of virus-specific TEM and TRM cells with polyfunctional cytokine capacity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pardieck, N. R. et al. (2021) A third vaccination with a single T cell epitope protects against SARS-CoV-2 infection in the absence of neutralizing antibodies. bioRxiv 2021.12.15.472838; doi: https://doi.org/10.1101/2021.12.15.472838, https://www.biorxiv.org/content/10.1101/2021.12.15.472838v1
- Peer reviewed and published scientific report.
Pardieck, Iris N., Tetje C. van der Sluis, Esmé T. I. van der Gracht, Dominique M. B. Veerkamp, Felix M. Behr, Suzanne van Duikeren, Guillaume Beyrend, et al. 2022. “A Third Vaccination with a Single T Cell Epitope Confers Protection in a Murine Model of SARS-CoV-2 Infection.” Nature Communications 13 (1): 3966. https://doi.org/10.1038/s41467-022-31721-6. https://www.nature.com/articles/s41467-022-31721-6.