Background
Global efforts to reduce the spread of SARS-CoV-2 have been mixed and profound, including both national and local lockdowns, contact tracing, and vaccinations. The reverse transcriptase-polymerase chain reaction (RT-PCR) test has been the most widely used diagnostic test around the globe to detect SARS-CoV-2.
However, rapid antigen (Ag) testing has been deployed to scale up the detection at the population level and facilitate access to coronavirus disease 2019 (COVID-19) tests in resource-poor regions to complement RT-PCR testing. The rapid antigen tests for COVID-19 detect the viral nucleocapsid protein, making antigen tests less sensitive than RT-PCR. However, these tests are less expensive than RT-PCR, provide rapid results, and are easily portable.
About the study
In the present study, researchers evaluated rapid antigen tests commercially used in Australia against the SARS-CoV-2 Delta variant. The team conducted a laboratory evaluation study at the Victorian Infectious Diseases Reference laboratory and assessed the analytical sensitivity and specificity of 22 antigen tests, as well as the clinical sensitivity and specificity of a high-performance antigen test, and correlated their results with infectivity in cell culture.
Analytical sensitivity was assessed against the SARS-CoV-2 Delta variant and reported as TCID50/mL, cycle threshold (Ct), and viral load (RNA copies/mL), whereas specificity was evaluated against non-SARS-CoV-2 viruses. For clinical sensitivity and correlation with cell culture infectivity, the researchers used the Abbott PanBioTM COVID-19 Ag test.
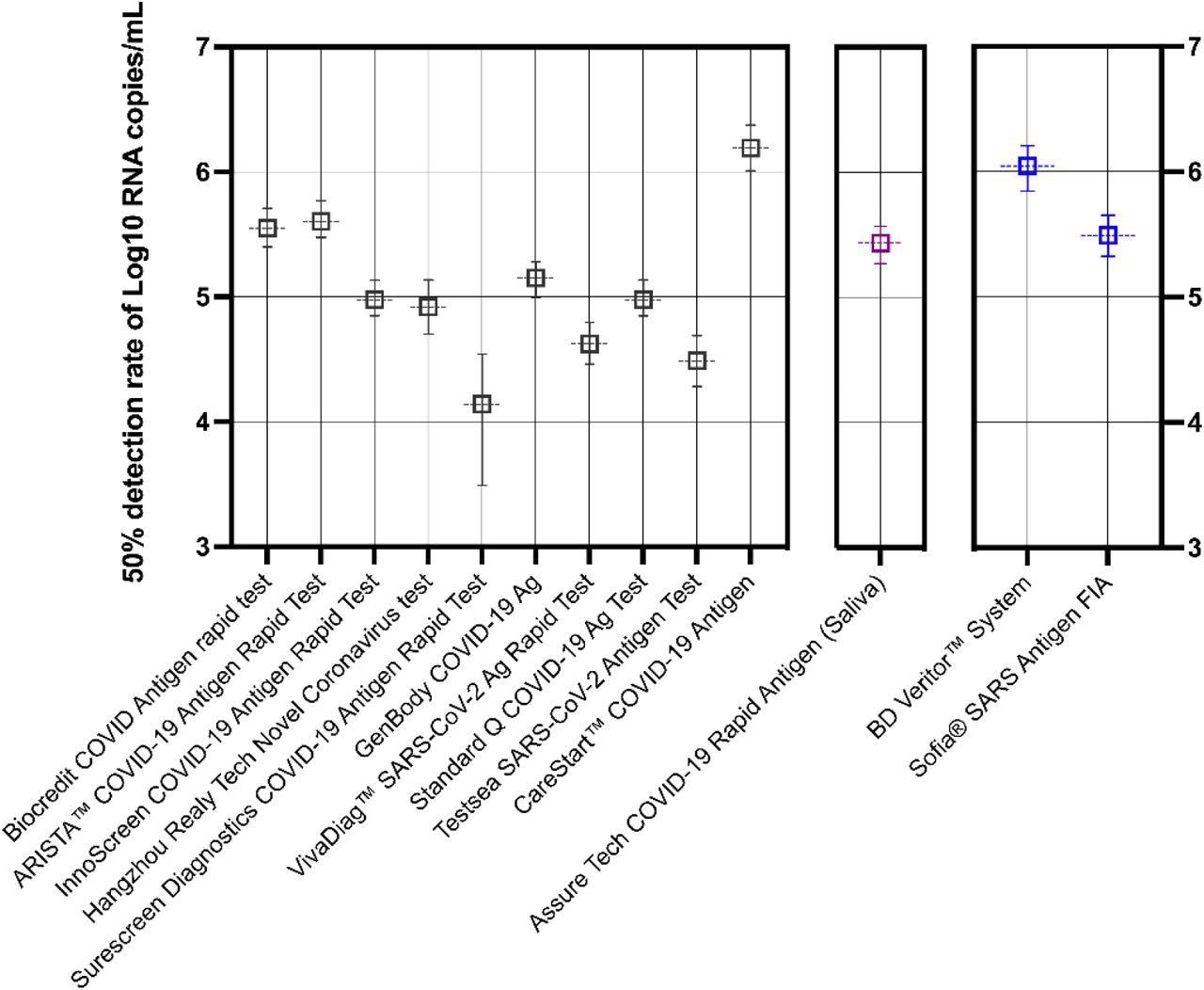
Predictive probability of rapid antigen test positivity against SARS-CoV-2 (B.1.617.2). Thirteen tests were analyzed by logistic regression and each result shown is the mean viral load (log10 RNA copies/mL) expressed as a 50% probability of positive antigen detection. Note that predictive probabilities could not be determined for some kits, where data showed perfect separation (clear distinction between a positive *1* and negative *1* result) and are not depicted above. Error bars represent the 95% confidence interval (CI). Lateral-flow devices (LFDs) are colored black, saliva-based antigen tests highlighted purple, and devices requiring a separate device for results interpretation in blue. The Sofia FIA test is a fluorescence-based test.
Study findings
The researchers found that 19 kits were consistent in detecting SARS-CoV-2 antigen equivalent to 1.3 x 106 copies/mL, while 11 kits demonstrated a 50% detection probability for viral loads greater than 106 RNA copies/mL. Three kits reported detection levels in ng/mL and were not compared.
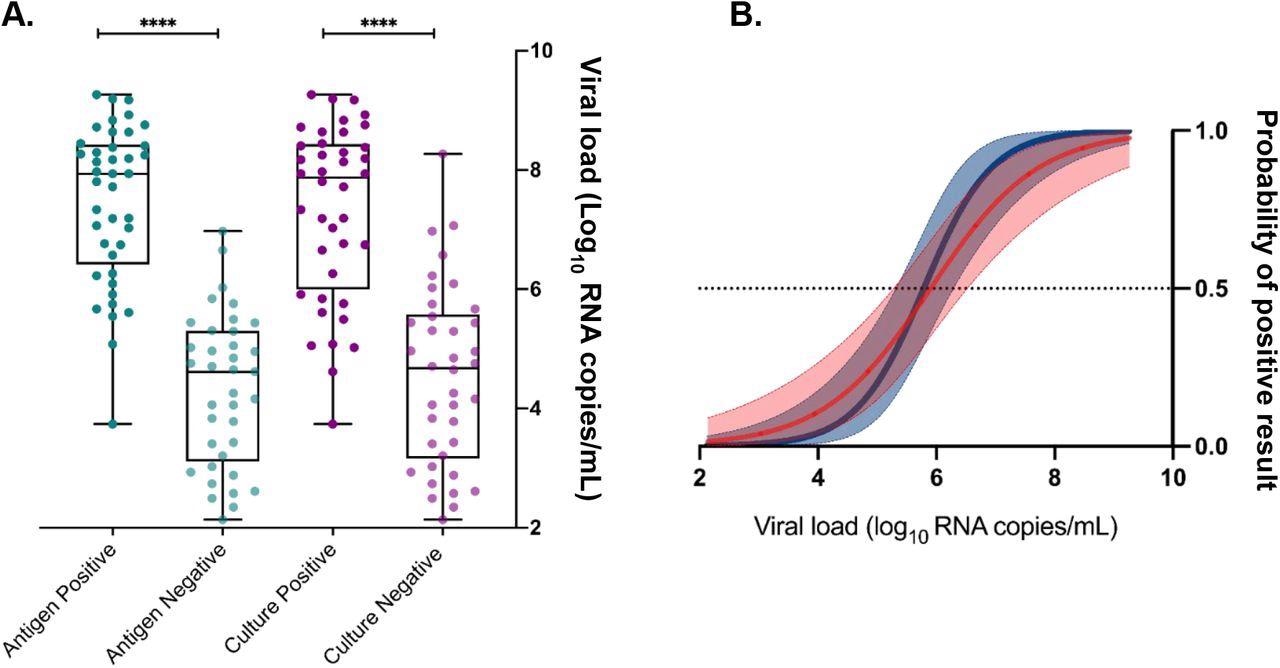
Assessment of viral load, antigen test positivity, and cell culture infectivity. Logistic regression models (A) were performed using viral load (log10 RNA copies/mL) to calculate the 50% probability of predicting a positive antigen test (dark blue) and positive recovery of infectious virus by cell culture (red). Ninety-five percent confidence intervals are shaded and the dotted line represents a 50% positive probability. Virus load in clinical samples (B) was significantly higher for a positive antigen result (dark green) and in samples that recovered infectious virus by cell culture (purple) compared to antigen (light green) and infectivity (light purple) negative samples. **** P-value <0.0001.
About 12 kits were less sensitive than their manufacturers’ claimed sensitivities. No cross-reactivity with other respiratory viruses was observed with any of the kits. The analytical sensitivity of the kits remained unchanged for SARS-CoV-2 samples irradiated with gamma rays.
The group analyzed 78 SARS-CoV-2 RT-PCR positive samples for the presence of Ag and reactivity using the Abbott PanBioTM COVID-19 Ag test. The findings quantified as viral RNA copies/mL had a median viral load of 4.62 x 105 RNA copies/mL. Of these 78 samples, 41 were positive in Ag tests, and 40 of these Ag positive specimens reported positive cell culture growth.
Additionally, the viral load was found to be significantly higher in positive cell cultures and Ag test positive samples than those in culture and Ag test negative samples. Clinical specificity was assessed by testing about 100 SARS-CoV-2 negative samples. All 22 kits reported a specificity of 100% compared with RT-PCR.
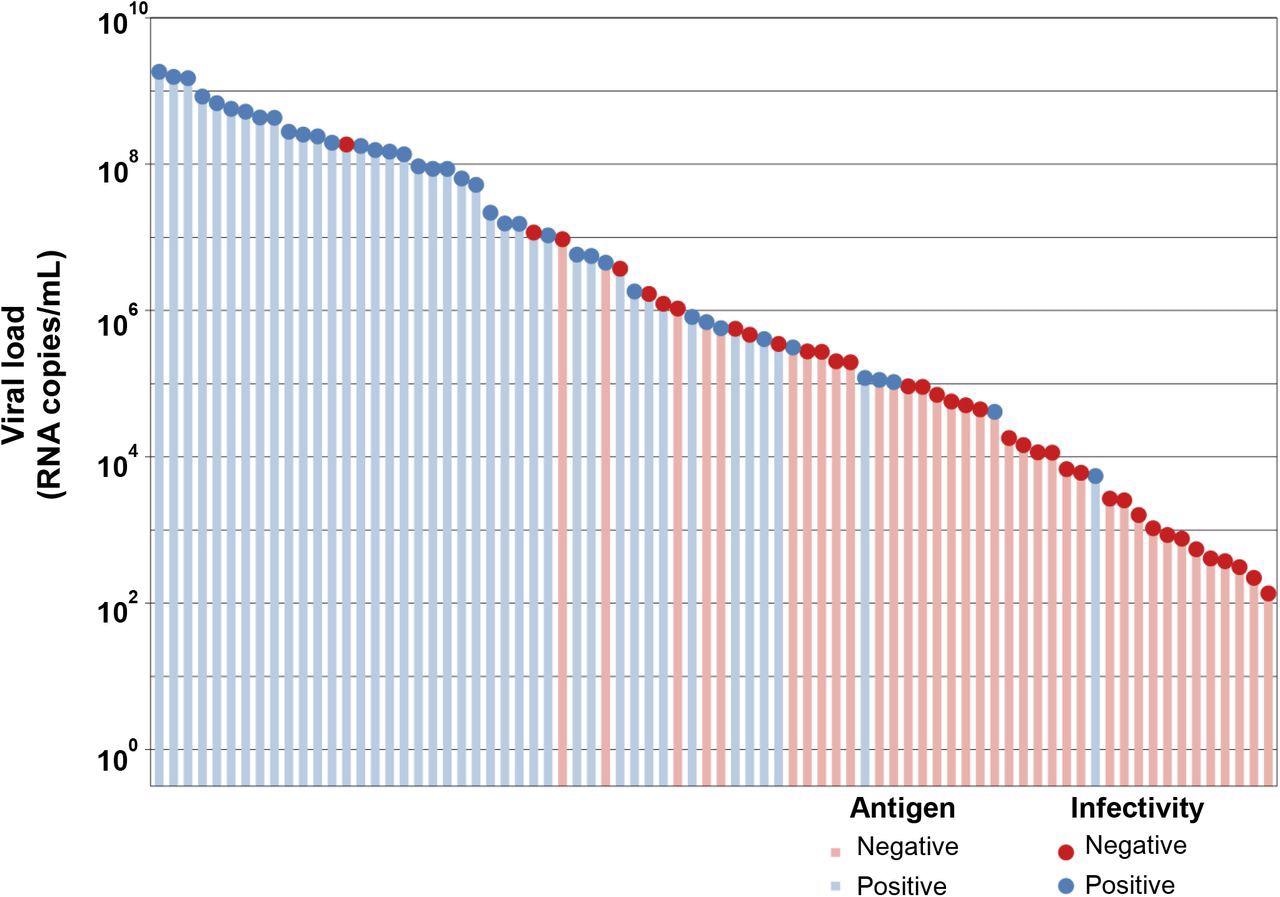
Correlation of antigen test positivity with tissue culture positivity. Clinical naso-oropharyngeal swab samples were submitted for SARS-CoV-2 RT-PCR testing using an in-house N-gene assay. Residual volume was tested for viral infectivity by cell culture (circles), and antigen testing using the Abbott PanBio ™ COVID-19 Ag test kit (bars). Colouring represents a positive sample (blue) or a negative sample (red).
Limitations
The present analysis was performed using the spiked virus in viral transport media (VTM) to evaluate Ag test kits instead of using swabs in the manufacturer-provided buffer. Further, the clinical sensitivity was assessed for only one Ag test known as the Abbott PanBioTM COVID-19 Ag test.
Conclusions
This laboratory evaluation study found that all tested 22 rapid antigen tests were able to detect Ags at a sensitivity of 1.2 x 104 TCID50/mL or 2.6 x 106 viral RNA copies/mL. The minimum standard recommended by the World Health Organization (WHO) is 102-103 TCID50/mL for Ag tests.
The present evaluation reported a detection range within the range of 8.3 x 104 to 2.6 x 106 RNA copies/mL. The team was also able to correlate Ag test positivity with Ct values, RNA copies/mL, and TCID50/mL.
The researchers demonstrated that Ag test positivity correlated with the positive viral culture, suggesting that Ag tests could be useful in determining SARS-CoV-2 transmission risk. However, several kits reported variations in sensitivities stated by manufacturers, thus necessitating the evaluation of analytical sensitivity before deployment for population-level testing.
Despite any inconsistencies, most kits were able to meet WHO standards for SARS-CoV-2 detection. The current study’s findings provide a basis to select and implement Ag tests by using standardized protocols.
The data obtained in this study also mandates post-market validation of all existing commercial Ag tests regularly to help guide the implementation strategy of these tests.
Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information