During the early phase of the coronavirus disease 2019 (COVID-19) pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections were less common in children. As a result, many were under the widely debated assumption that children were less susceptible to SARS-CoV-2 infection.
Since late 2019, the global understanding of the epidemiology of SARS CoV-2 infection in children has evolved. Infections have been observed to be milder in children, thus suggesting that immune responses vary with age.
In a new study published on the medRxiv* preprint server, scientists compared receptor binding domain antibodies (RBDAb) and SARS-CoV-2 neutralizing antibodies (neutAb) in children and adults in a SARS-CoV-2 household study. To this end, the researchers found that children mount robust antibody responses to SARS-CoV-2 following community infections.
.jpg)
Study: Binding and Neutralizing Antibody Responses to SARS-CoV-2 in Infants and Young Children Exceed Those in Adults. Image Credit: maxbelchenko / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
There is no conclusive evidence of whether children mount more robust SARS-CoV-2 antibody responses than adults. Moreover, information on the immune responses of children aged 0-4 years against SARS-CoV-2 is especially limited.
Evaluation of several vaccines in children aged 0-4 years is ongoing; however, vaccines are currently recommended for children aged 5-17 years in the United States. Assessment of the magnitude and quality of antibody responses to SARS-CoV-2 infection in very young children is essential, as this could inform public health policies and decisions to approve COVID-19 vaccines in this age group.
About the study
Scientists conducted a SARS-CoV-2 Epidemiology And Response in Children (SEARCh) study to address SARS-CoV-2 susceptibility, illness, transmission, and immunologic responses in children and their adult household members. Herein, titers of SARS-CoV-2 binding and neutAb against both the SARS-CoV-2 wild-type (wt) and Delta strains were assessed. The children included in the current study were aged 5-17 years and 0-4 years.
Sera were collected from a total of 682 SEARCh participants in 175 households, among which 49% were adults, 14% were children aged 5-17 years, and 37% were children aged 0-4 years. RBDAb)in sera was detected in 55 participants, including 27 RBDAb seropositive children. No participant was hospitalized with COVID-19 before enrollment.
Study findings
The scientists observed that in the subset of 55 participants who were RBDAb seropositive, the geometric mean titer (GMT) of RBDAb was higher in children aged 0-4 years by ten-fold as compared to adults. When neutAb were assessed, those generated against the SARS-CoV-2 wt strain in children aged 0-4 and 5-17 years showed similar pseudovirus viral neutralizing antibody (PsVNA) 50% inhibitory dilution (ID50) titers.
In fact, PsVNA ID50 titers were double in children aged 0-17 years as compared to adults. Children aged 0-4 years had the highest PsVNA ID50 titers of all seropositive individuals across a number of households.
PsVNA ID50 GMTs to the Delta variant of SARS-CoV-2 were comparatively modest in all age groups. Furthermore, the ratio of titers to the wt and Delta strains of SARS-CoV-2 did not differ between age groups.
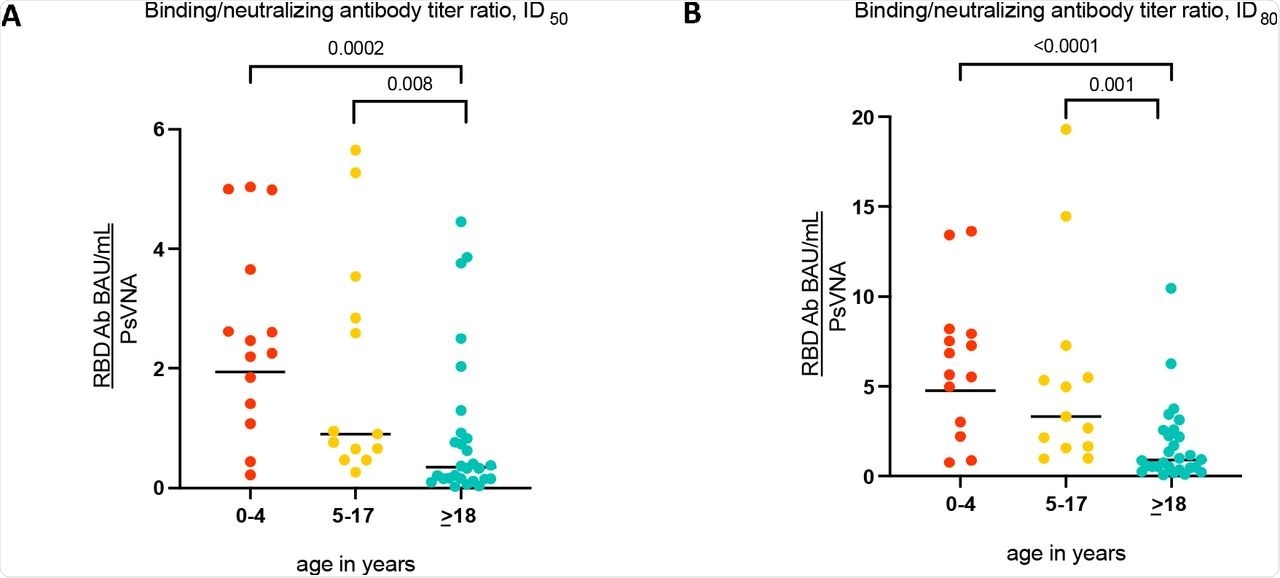 GMTR of SARS-CoV-2 RBDAb (BAU/mL) to SARS-CoV-2 PsVNA (orange= children 0-4 years, yellow= children 5-17 years, blue=adults 18-62 years).
GMTR of SARS-CoV-2 RBDAb (BAU/mL) to SARS-CoV-2 PsVNA (orange= children 0-4 years, yellow= children 5-17 years, blue=adults 18-62 years).
When assessing individuals with serologic evidence of prior SARS-CoV-2 infection, children between the ages of 0-4 years showed about 10-fold higher levels of RBDAb and about two-fold higher levels of neutAb to the wt SARS-CoV-2 strain as compared to adults. The robustness of the results across households proves that the observed differences were unrelated to the timing of infection. Further, these differences are not likely to be driven by the differences in illness severity, as many children had no known history of COVID-19.
To date, a limited number of studies have compared the antibody responses to SARS-CoV-2 in children and adults. The current study helps address this research gap by providing more data for children aged 0-4 years, which is an understudied population.
Further, the current study also shows that the differences in the amount of the RBDAb titers are consistent within households, in which the timing of infections is likely similar. Young children also appear to make proportionally more SARS-CoV-2 RBDAb than neutAb as compared to adults.
Limitations
The first limitation of the current concerns the sample size, in that the number of SARS-CoV-2 RBDAb positive subjects was relatively small. Secondly, the results largely reflect immune responses to the original SARS-CoV-2 strain and may not be generalizable to cases with emerging variants.
The third limitation concerns the cross-sectional nature of the study, which might not account for differences in the timing of infection between adults and children. Future research should consider more longitudinal studies with larger sample sizes and include individuals in whom the timing of infection and type of SARS-CoV-2 variant involved in the infection is known.
Conclusion
In the current study, scientists have demonstrated that young children between the ages of 0-4 years)are capable of mounting substantial RBD binding and neutralizing Ab responses as compared to adults in the same households.
The results support the use of a reduced dose of the BNT162b2 vaccine in an ongoing trial in young children aged 0-4 years. The second implication of this study is that RBDAb responses may not be a good predictor of neutAb responses in young children.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Karron, A. R., Quesada, M. G., Schappell, E. A., et al. (2021) Binding and Neutralizing Antibody Responses to SARS-CoV-2 in Infants and Young Children Exceed Those in Adults. medRxiv. doi:10.1101/2021.12.20.21268034. https://www.medrxiv.org/content/10.1101/2021.12.20.21268034v1.
- Peer reviewed and published scientific report.
Karron, Ruth A., Maria Garcia Quesada, Elizabeth A. Schappell, Stephen D. Schmidt, Maria Deloria Knoll, Marissa K. Hetrich, Vic Veguilla, Nicole Doria-Rose, and Fatimah S. Dawood. 2022. “Binding and Neutralizing Antibody Responses to SARS-CoV-2 in Very Young Children Exceed Those in Adults.” JCI Insight 7 (8). https://doi.org/10.1172/jci.insight.157963. https://insight.jci.org/articles/view/157963.