
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
SARS-CoV-2, which is the etiological agent of the coronavirus disease 2019 (COVID-19), has undergone several mutations that have led to the emergence of numerous variants around the world. Despite high COVID-19 vaccine coverage in many nations, multiple breakthrough SARS-CoV-2 infections have still been reported in these countries.
In November 2021, the SARS-CoV-2 Omicron (B.1.1.529) variant was initially identified. Soon after its initial discovery, Omicron quickly began replacing the highly transmissible Delta variant (B.1.617.2) as the dominant circulating strain in many countries around the world.
As compared to the original strain of SARS-CoV-2, the Omicron variant consists of two deletions, one insertion, and 30 amino acid differences in its viral spike protein. Notably, many of the mutations found in the Omicron variant have previously been identified to or confer resistance to neutralizing antibodies.
Extensive research regarding the antigenic shift and pathogenesis of the Omicron variant, as well as the ensuing human immune response, is currently being conducted. Due to the rapid spread of the Omicron variant across the world, despite vaccinations and mitigation protocols, it is crucial to understand the potential ability of this new strain of SARS-CoV-2 to evade neutralizing antibodies that are elicited by both vaccines and therapeutic monoclonal antibodies.
About the study
Using a pseudotyped virus assay, the researchers in the current study characterized the sensitivity of the Omicron variant neutralization by a diverse set of samples. These samples included relevant monoclonal antibodies, pooled serum from vaccine recipients, serum samples from infected and infected-then-vaccinated healthcare workers, as well as a random sample of recent seropositive blood donors.
Study findings
The researchers evaluated the sensitivity of the Omicron variant to neutralization by several monoclonal antibodies currently being used to treat COVID-19 patients, including REGN10933, REGN10987, Ly-CoV016, Ly-CoV555, and S309, the latter of which was a parent antibody of Sotrovimab. Unfortunately, the Omicron variant was resistant to neutralization by REGN10933, REGN10987, Ly-CoV016, and Ly-CoV555, even when the highest concentration of 10 micrograms (μg)/ml was tested. However, S309 was found to retain much of its neutralizing activity against the variant, despite experiencing a two-fold loss in its potency.
Antibodies elicited by a prior SARS-CoV-2 infection with the original wild-type strain of the virus exhibited a reduced neutralization against the Omicron variant. This reduction in neutralization was estimated to be 40-fold as compared to that which was achieved against the wild-type SARS-CoV-2 variant.
Further, neutralizing antibody responses shortly after infection or vaccination with either the Pfizer/BioNTech BNT162b2, Moderna mRNA-1273, or Johnson & Johnson Ad26.COV2.S vaccines were substantially less potent against the Omicron variant. This reduction in neutralization ranged from 7- to 45-fold across all serum pools.
Samples that were obtained from infected-then-vaccinated individuals presented robust neutralizing potency against the Omicron variant. The significant neutralization of variants in these individuals demonstrates the utility of vaccination, particularly in individuals who were previously infected with SARS-CoV-2.
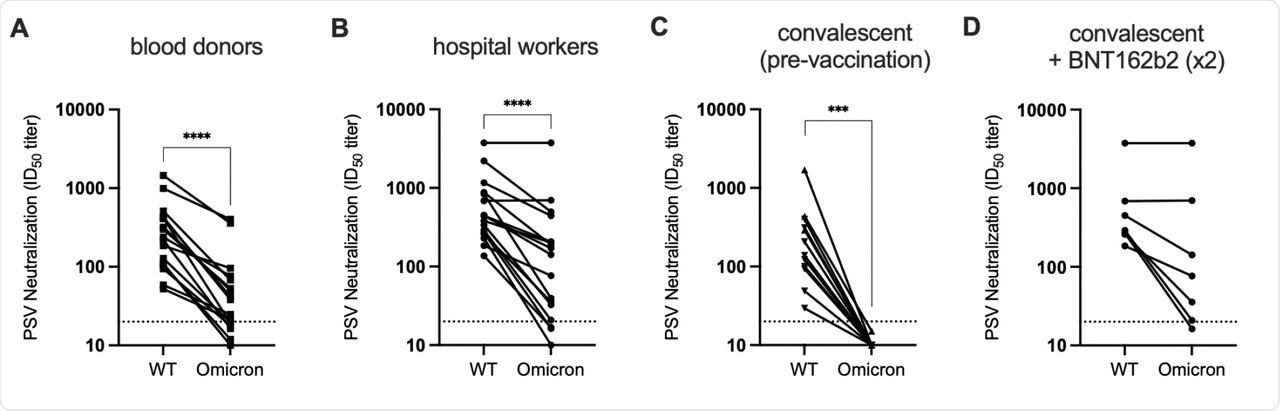
Characterization of the relative neutralization resistance of Omicron. A-B.
Conclusion
Overall, the data presented in the current study demonstrates an extensive but incomplete loss of neutralization against the Omicron variant by both natural and vaccine-induced antibodies, as well as current therapeutic monoclonal antibodies. These findings demonstrate that in light of the current situation where Omicron is the dominant circulating strain, previously infected individuals who are unvaccinated cannot necessarily be considered immune against this new strain of SARS-CoV-2. However, vaccination of individuals with a history of COVID-19 provides impressive cross-neutralizing antibody responses that can offer some protection against the Omicron variant.
Although the S309 antibody retained some of its neutralization against the Omicron variant, most of the monoclonal antibodies that are currently being used in the clinical setting were unable to neutralize this new strain of SARS-CoV-2. Therefore, the researchers argue that rapid diversification of current therapeutic monoclonal antibodies is needed in order to account for potency losses against the Omicron variant, as well as future variants of SARS-CoV-2.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sheward, D. J., Kim, C., Ehling, R. A., et al. (2021). Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron). bioRxiv. doi:10.1101/2021.12.19.473354. https://www.biorxiv.org/content/10.1101/2021.12.19.473354v1.full.
- Peer reviewed and published scientific report.
Sheward, Daniel J., Changil Kim, Julian Fischbach, Kenta Sato, Sandra Muschiol, Roy A. Ehling, Niklas K. Björkström, et al. 2022. “Omicron Sublineage BA.2.75.2 Exhibits Extensive Escape from Neutralising Antibodies.” The Lancet Infectious Diseases 22 (11): 1538–40. https://doi.org/10.1016/S1473-3099(22)00663-6. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00663-6/fulltext.