Whereas the variant completely escapes the neutralizing activity from doubly vaccinated individuals, convalescent individuals, and different monoclonal antibodies in clinical use, the researchers from Germany showed that a booster vaccine robustly induces neutralization against the immune evasive Omicron variant.

Study: mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Image Credit: Tobias Arhelger/Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The Omicron variant (BA.1 sublineage of B.1.1.529) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in the Gauteng province in South Africa. It was designated as a variant of concern (VoC) by the World Health Organization (WHO) on 26 November 2021.
The Omicron variant exhibits potent immune evasion properties, as evident from the increased Omicron infections and re-infections in populations with a high prevalence of SARS-CoV-2 immunity.
Most COVID-19 vaccines are based on the viral spike (S) glycoprotein as the epitope to induce immunity against SARS-CoV-2. Mutations in these epitopes can result in reduced potency of the neutralizing antibodies. It is also associated with reduced vaccine effectiveness and impaired therapeutic activity of monoclonal antibodies.
Compared to the previous VoCs, there is an increased number of non-synonymous mutations in the Omicron variant relative to the ancestral Wu01 strain of the SARS-CoV-2, first identified in Wuhan, China, in late December 2019. Most of these mutations are in the viral spike glycoprotein, mainly covering the critical epitopes for the SARS-CoV-2-neutralizing antibodies.
Because these mutations improve the SARS-CoV-2’s resistance to immunity, studying the Omicron variant’s response has important implications in mitigating the COVID-19 pandemic.
Towards this goal, the present study looks at the Omicron variant and the neutralizing capacity of vaccinated individuals and convalescent individuals and the monoclonal antibody activity against Omicron.
Study findings
The researchers analyzed the susceptibility of the Omicron variant to vaccine-induced serum activity by testing individuals who had no prior infection but had completed two doses of the BNT162b2 vaccine.
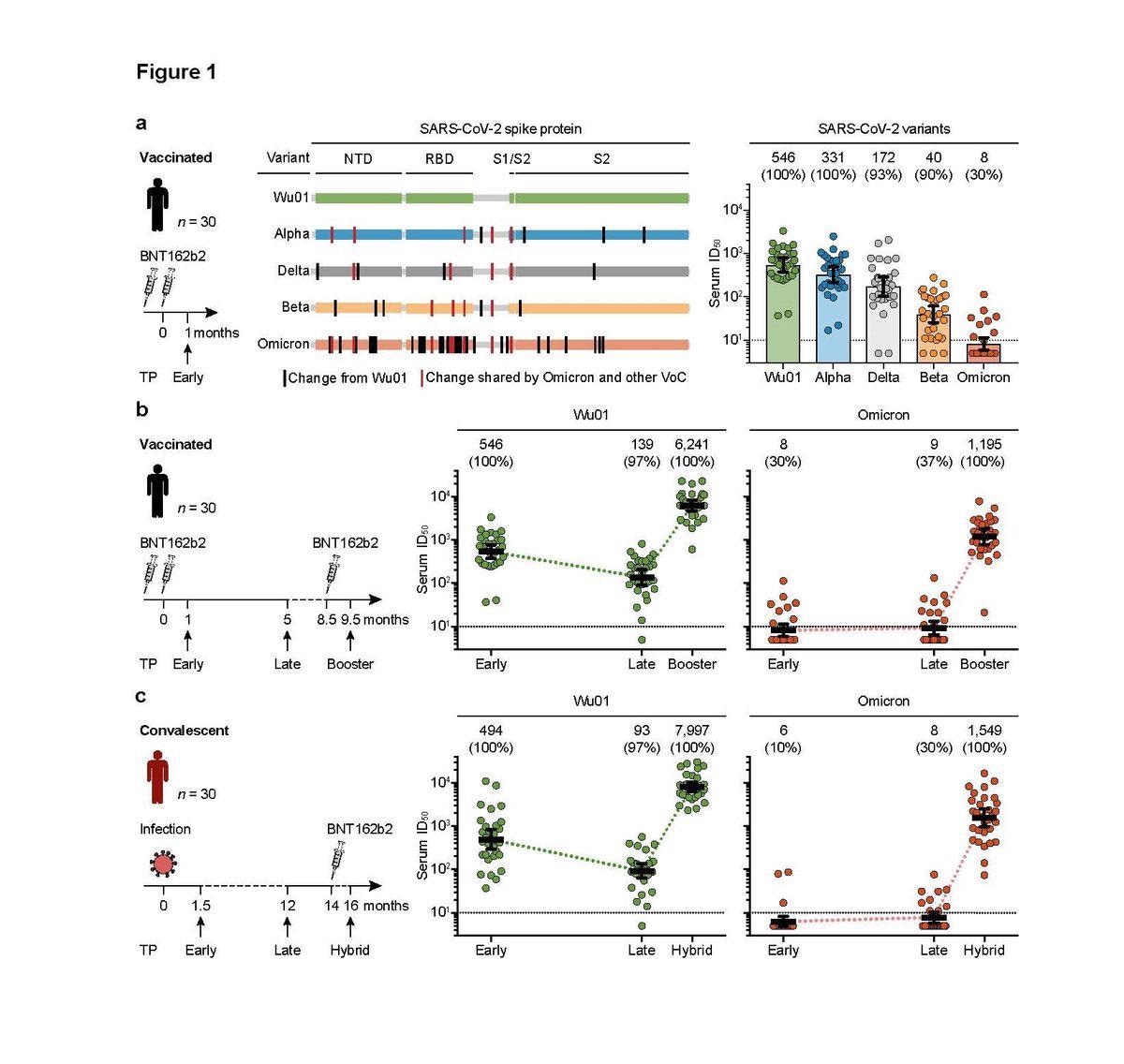
SARS-CoV-2-neutralizing serum activity in vaccinated and convalescent individuals. a, Neutralizing serum activity was determined in samples obtained one month after two doses of BNT162b2 against the ancestral Wu01 strain of SARS-CoV-2 and four variants of concern. Residues with changes relative to the Wu01 strain are indicated by intersecting lines. Fifty-percent inhibitory serum dilutions (ID50s) were determined by pseudovirus neutralization assays. Bars indicate geometric mean ID50s with 95% confidence intervals (CIs). b, Serum ID50s against the Wu01 strain (green) and the Omicron variant (red) of SARS-CoV-2 in a longitudinal cohort of 30 individuals. Samples were collected at a median of one month (Early, see a) and five months (Late) after two doses of BNT162b2, and one month after a subsequent single dose of BNT162b2 (Booster). Colored lines connect geometric mean ID50s and error bars indicate 95% CIs. c, Serum ID50s against the Wu01 strain (green) and the Omicron variant (red) of SARS-CoV-2 in a longitudinal cohort of 30 COVID-19-convalescent individuals. Samples were collected at a median of 1.5 (Early) and 12 months (Late) after diagnosis of SARS-CoV-2 infection, as well as 1.5 months after a single BNT162b2 vaccination (Hybrid). Colored lines connect geometric mean ID50s and error bars indicate 95% CIs. In a, b, and c, ID50s below the lower limit of quantification (LLOQ, ID50 of 10; indicated by black dashed lines) were imputed to ½ LLOQ (ID50=5). Numbers above graphs indicate geometric mean ID50s and the percentage of samples with detectable neutralizing activity above the LLOQ in parentheses. TP, time point; NTD, N-terminal domain; RBD, receptor-binding domain.
Using an established lentivirus-based pseudovirus assay, they found the serum neutralizing activity to be decreasing in the virus tested: Wu01 strain, the Alpha (B.1.1.7), Delta (B.1.617.2), and Beta (B.1.351), showed geometric mean 50% inhibitory serum dilution (GeoMean ID50) of 546, 331, 172, and 40, respectively.
Notably, out of the 30 vaccinated individuals tested, only eight displayed detectable levels of serum neutralizing activity (GeoMean ID50 of 8) against the Omicron variant. This activity is significantly lower than against the Beta variant, which previously was one of the most evasive variants.
Investigating the longitudinal changes in serum neutralizing activity against the Omicron variant, the researchers tested 30 vaccinees at five months after BNT162b2 vaccination, as well as one month after a single BNT162b2 booster dose.
In the case of the two-dose BNT162b2 vaccine, they found that the neutralizing activity against the Wu01 decreased four-fold with time from initial levels but strongly soared up after booster vaccination (GeoMean ID50 of 6,241).
The neutralizing activity against the Omicron variant was found in a few vaccinated individuals; it remained extremely low and stable (geometric mean ID50 of 8 and 9). However, interestingly, upon booster vaccination, the neutralizing serum activity against the Omicron variant increased over 100-fold (geometric mean ID50 of 1,195), which was detectable in all 30 participants.
To evaluate the long-term immune response to the Omicron variant in COVID-19 convalescent individuals, the researchers collected serum samples at different time points from individuals who were infected with SARS-CoV-2. Additionally, they collected samples after the administration of a single dose of BNT162b2, resulting in a “hybrid immunity” - acquired by a combination of infection and vaccination.
While the neutralizing activity was variable in the convalescent individuals against Wu01 and increased after vaccination, there was no neutralization against the Omicron variant. Interestingly, however, the researchers observed a slight increase in some convalescent individuals at late time points, indicative of a potential ‘ongoing affinity maturation resulting in antibodies capable of targeting a wider spectrum of SARS-CoV-2 variants.’
However, despite zero neutralizing activity against the Omicron variant after a SARS-CoV-2 infection, a single dose of BNT162b2 induced a strong response (GeoMean ID50 of 1,549).
Further, the researchers determined the SARS-CoV-2-neutralizing activity of the monoclonal antibodies, which are demonstrated to have effectively reduced COVID-19-related morbidity and mortality. The antibodies tested in the present study are Bamlanvimab, Etesevimab, REGN10933 (casirivimab), REGN10987 (imdevimab), C102, P2B-2F6, and S309 (sotrovimab), Fab2-36, and an antibody in clinical investigation - DZIF-10c.
As IC50 values >10 µg/ml indicate failure to achieve 50% neutralizing activity at the highest tested antibody concentration of 10 µg/ml, the results showed that only two antibodies presented some activity against the Omicron variant. The Wu01 and the Alpha variant were susceptible to all the antibodies whereas, the Delta and Beta variants were neutralized by most of the antibodies.
Conclusions
This new Omicron variant of SARS-CoV-2 is causing a rapid increase in infections across the globe as it escapes the humoral immune response in BNT162b2-vaccinated and convalescent individuals. Because of the unusually high number of mutations in key epitopes of the neutralizing antibodies on the viral spike glycoprotein, the potential immune evasion is substantial in the Omicron variant.
The present study assessed the serum neutralizing capacity in longitudinal cohorts of vaccinated and convalescent individuals, and booster immunized, as well as monoclonal antibody activity against Omicron using pseudovirus neutralization assays.
The study found a near-complete lack of neutralizing activity against the Omicron variant in vaccinated individuals (two doses of the BNT162b2 COVID-19 vaccine) and in convalescent individuals, as well as resistance to different monoclonal antibodies in clinical use. Because most clinically used monoclonal antibodies had reduced neutralizing activity against the Omicron variant, the treatment option for Omicron-induced COVID-19 is now a challenge.
Significantly, the booster immunizations in vaccinated and convalescent individuals resulted in high neutralizing activity against Omicron, indicating that booster immunizations may help improve the humoral immune response against the Omicron variant.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Henning Gruell, Kanika Vanshylla, Pinkus Tober-Lau et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant, 27 December 2021, PREPRINT (Version 1) available at Research Square https://doi.org/10.21203/rs.3.rs-1168453/v1, https://www.researchsquare.com/article/rs-1168453/v1
- Peer reviewed and published scientific report.
Gruell, Henning, Kanika Vanshylla, Pinkus Tober-Lau, David Hillus, Philipp Schommers, Clara Lehmann, Florian Kurth, Leif E. Sander, and Florian Klein. 2022. “MRNA Booster Immunization Elicits Potent Neutralizing Serum Activity against the SARS-CoV-2 Omicron Variant.” Nature Medicine, January, 1–4. https://doi.org/10.1038/s41591-021-01676-0. https://www.nature.com/articles/s41591-021-01676-0.