Scientists from the US and Germany have recently investigated the efficacy of the Pfizer-BioNTech mRNA-based coronavirus disease 2019 (COVID-19) vaccine in neutralizing the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The findings reveal that the Omicron neutralizing titers induced by the third vaccine dose are comparable to that achieved against the original Wuhan strain (wild-type SARS-CoV-2) by the two-dose regimen. The study is currently available on the medRxiv* preprint server while awaiting peer review.
Study: Neutralization of SARS-CoV-2 Omicron pseudovirus by BNT162b2 vaccine-elicited human sera. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The Omicron variant is the most recently emerged variant of SARS-CoV-2, which was detected for the first time in South Africa in November 2021. Because of significantly increased transmissibility, Omicron soon became the predominantly circulating variant in many countries across the globe. On November 26, the World Health Organization designated Omicron as the variant of concern (VOC).
The Omicron variant is heavily mutated compared to other identified VOCs. It has around 39 mutations in the spike protein, with 15 specifically in the receptor-binding domain (RBD) and 8 in the N-terminal domain (NTD). Preliminary studies have found that many of these mutations are located at the immunodominant epitopes of neutralizing antibodies induced by natural infection or vaccination. This has raised concerns that currently available COVID-19 vaccines may have reduced efficacy against the Omicron variant.
In the current study, the scientists have investigated the neutralizing potency of antibodies induced by the mRNA-based COVID-19 vaccine against the wild-type SARS-CoV-2 and its variants beta, delta, and Omicron.
Study design
The scientists used pseudoviruses expressing the spike protein of wild-type SARS-CoV-2 and beta, delta, and Omicron variants for neutralization. They used serum samples collected from vaccine recipients for estimating neutralizing titers against tested variants.
Among recipients, 32 received two doses, and 30 received three doses of the BNT162b2 mRNA vaccine developed by Pfizer/BioNTech. The recipients of the two-dose vaccination regimen provided serum samples 21 days post the second dose. The recipients of the three-dose vaccination regimen provided samples one month after the third dose. The recipients received the third dose more than six months after receiving the second dose.
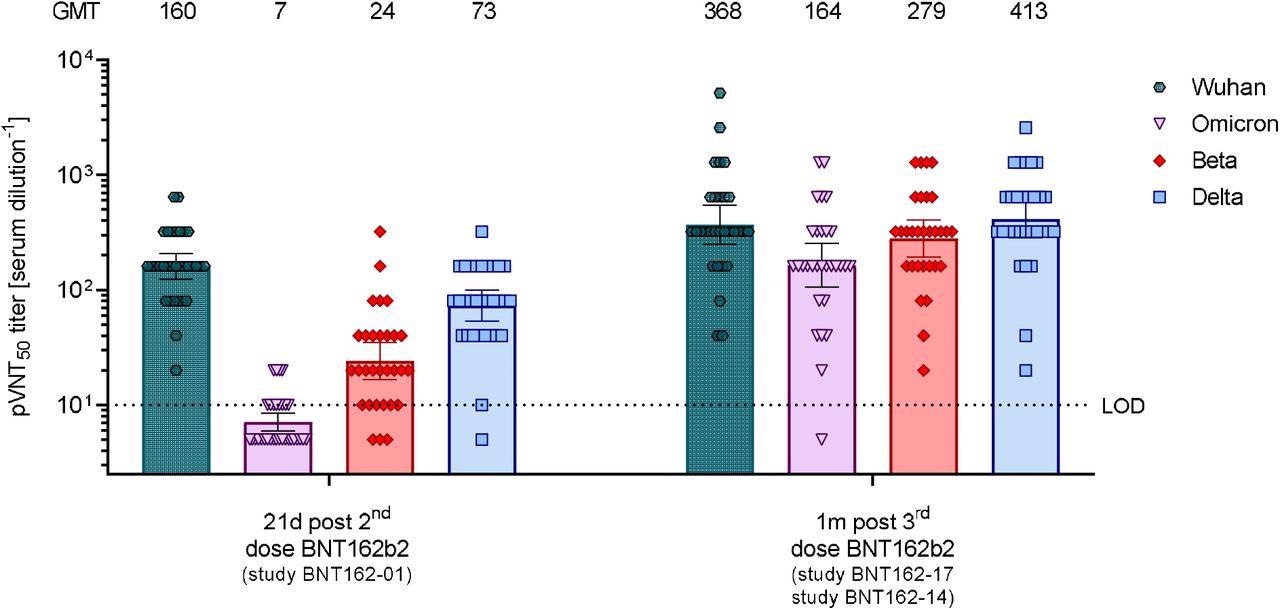
50% pseudovirus neutralization titers (pVNT50) of sera from vaccine recipients collected after two or three doses of BNT162b2 against VSV-SARS-CoV-2-S pseudovirus bearing the Wuhan, Omicron, Beta, or Delta variant spike protein. N=32 sera from participants in study BNT162-01 drawn at 21 days after dose 2, and n=30 sera from participants in the BNT162-14 (n=11) and BNT162-17 trials (n=19) drawn at 1 month after dose 3 were tested. Each serum was tested in duplicate and geometric mean 50% pseudovirus neutralizing titers (GMTs) were plotted. For values below the limit of detection (LOD), LOD/2 values were plotted. Group GMTs (values) and 95% confidence intervals are indicated.
Important observations
Efficacy of two-dose regimen
The antibodies induced by the two-dose regimen showed a 22-fold lower neutralizing efficacy against the Omicron variant compared to that against the wild-type virus. While most of the two-dose-vaccinated serum samples neutralized the beta and delta variants, only 12 out of 32 samples showed detectable titers against the Omicron variant. However, the neutralizing titers were 6-fold and 2-fold lower against the beta and delta variants compared to the wild-type virus, respectively.
Efficacy of three-dose regimen
The third vaccine dose caused a 23-fold induction in neutralizing titers against the Omicron variant compared to that obtained after the second vaccine dose. The titers were comparable to that achieved against wild-type virus after the second dose. Importantly, almost all samples (29 out of 30 samples) showed neutralizing efficacy against the Omicron variant. The third dose also increased the neutralizing titers against wild-type SARS-CoV-2 and the beta and delta variants.
Cellular immune responses
The scientists analyzed a panel of class I human leukocyte antigen (HLA) restricted spike-specific T cell epitopes to assess whether the Omicron variant can escape host cellular immune responses induced by vaccination.
The findings revealed that about 85% of tested T cell epitopes remained unaffected by the spike mutations observed in the Omicron variant. This observation indicates that the overall T cell responses induced by vaccination may still be effective against the Omicron variant.
Study significance
The study highlights the importance of the third vaccine dose in effectively boosting neutralizing titers against Omicron infection. Although it effectively neutralizes the beta and delta variants, the two-dose vaccination regimen may have significantly lower efficacy against the Omicron variant.
As mentioned by the scientists, further studies are required to assess immunogenicity and durability of protection provided by the third vaccine dose in real-world pandemic setups.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Muik A. 2021. Neutralization of SARS-CoV-2 Omicron pseudovirus by BNT162b2 vaccine-elicited human sera, medRxiv, https://www.medrxiv.org/content/10.1101/2021.12.22.21268103v1
- Peer reviewed and published scientific report.
Muik, Alexander, Bonny Gaby Lui, Ann-Kathrin Wallisch, Maren Bacher, Julia Mühl, Jonas Reinholz, Orkun Ozhelvaci, et al. 2022. “Neutralization of SARS-CoV-2 Omicron by BNT162b2 MRNA Vaccine–Elicited Human Sera.” Science 375 (6581): 678–80. https://doi.org/10.1126/science.abn7591. https://www.science.org/doi/10.1126/science.abn7591.