Monoclonal antibodies are gaining traction as powerful tools for preventing infectious diseases. Recently, the rate of antibody discovery has accelerated, and the Coronavirus disease 2019 (COVID-19) pandemic has highlighted the importance of these antibodies in combating pathogens such as severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2). There is, however, still a question about the utility of monoclonal antibodies against SARS‑CoV‑2, especially when there are already effective vaccines available.
Joshua Tan of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health wrote a review article in the journal DNA and Cell Biology about the role of monoclonal antibodies and briefly described a study on bispecific antibodies that neutralize SARS-CoV-2. The research group has generated bispecific antibodies to target different regions within the spike protein, the virus' main target on its surface. Several of these antibodies have been found to be highly effective at neutralizing SARS-CoV-2 in both cell culture experiments and animal experiments. Because these bispecific antibodies target two different sites on the spike protein, they will be less affected by mutations that alter the shape of a single site. Indeed, two of these antibodies have demonstrated complete functionality against the alpha, beta, gamma, and delta variants.
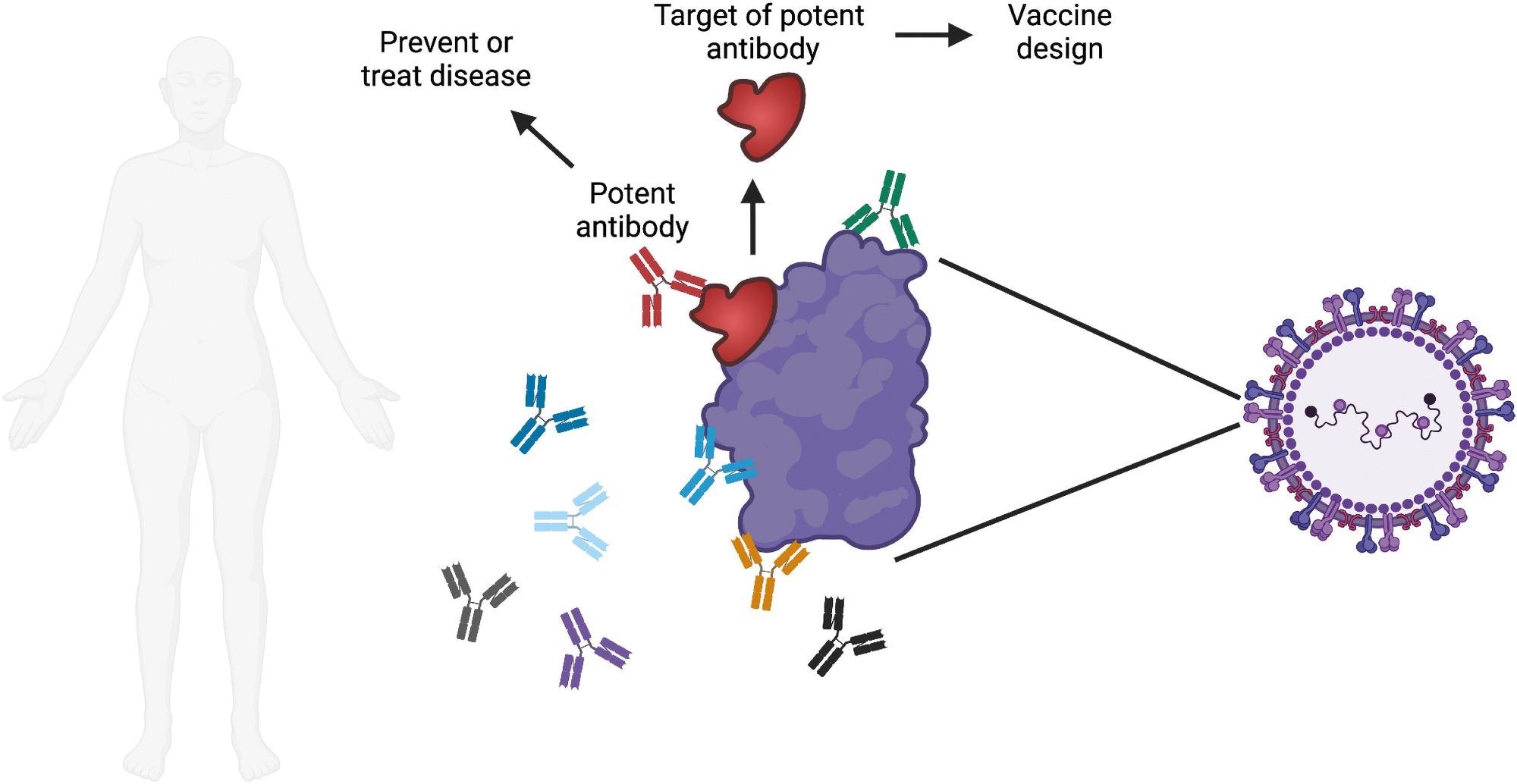 Overview of monoclonal antibody research. Each person makes many different antibodies in response to infection or vaccination. One major goal of monoclonal antibody research is to identify the most potent antibodies against a specific pathogen of interest. Potent antibodies can be tested for the ability to prevent or treat disease in humans. The site on the pathogen that is bound by the potent antibody can be evaluated for use in a vaccine, with the goal of triggering the production of these potent antibodies when the vaccine is administered. Image created with Biorender.com.
Overview of monoclonal antibody research. Each person makes many different antibodies in response to infection or vaccination. One major goal of monoclonal antibody research is to identify the most potent antibodies against a specific pathogen of interest. Potent antibodies can be tested for the ability to prevent or treat disease in humans. The site on the pathogen that is bound by the potent antibody can be evaluated for use in a vaccine, with the goal of triggering the production of these potent antibodies when the vaccine is administered. Image created with Biorender.com.
The study
As evidenced in the coronavirus disease 2019 (COVID-19) pandemic, vaccines that work well are the most efficient strategy to avoid infectious disease in most cases. Successful vaccines function by priming the immune system to fight a specific infection, usually by inducing potent antibodies against it. As a result, when the virus comes, it is met by an army of antibodies (and immune cells) ready to neutralize it before establishing a foothold and spreading throughout the body. One of the most critical functions of monoclonal antibodies, such as vaccinations, is to act as a disease preventative agent.
The employment of monoclonal antibodies during the COVID-19 epidemic is an example that connects many of the themes already mentioned. This pandemic has demonstrated the destruction that an unrestrained infectious pathogen can cause. Since the start of the pandemic, various research laboratories have been working hard to find effective monoclonal antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This endeavor has been seen as a supplement to vaccines, and it has continued in full force even after many COVID-19 vaccines have been proven to be highly effective.
The scientific community has produced a significant number of powerful neutralizing antibodies against SARS-CoV-2 at breakneck speed, including three medicines approved by the US Food and Drug Administration for the treatment of mild-to-moderate COVID-19 patients at risk of advancing to more severe disease. COVID-19–related hospitalization and fatalities have been observed to be reduced by these antibodies. During the course of infectious disease, monoclonal antibodies may be helpful in downregulating a pathogenic immune response. Tocilizumab, an antibody that targets the immunological protein IL-6, is currently being utilized to treat severe COVID-19 cases.
The effectiveness of these monoclonal antibodies against the variations of concern that have appeared in the last year has been a major source of concern. To address this issue, the authors developed bispecific antibodies that attack separate areas of the SARS-CoV-2 spike protein, the virus's principal target on the surface. Several of these antibodies have been demonstrated to be particularly successful at neutralizing SARS-CoV-2 in cell culture and preventing illness in an animal model.
These bispecific antibodies will be less influenced by mutations that modify the shape of a single site because they target two separate sites on the spike protein, and two of them have been proven to be fully effective against the alpha, beta, gamma, and delta versions. As new variants develop, the scientific community will continue to examine the available pool of antibodies to ensure that they are still effective against them.
Implications
Three lethal coronaviruses, an influenza pandemic, and many Ebola outbreaks have all occurred in the twenty-first century. Unfortunately, this trend is likely to continue with more encounters with wildlife carrying unknown infections and the rise of worldwide travel, which enables the quick transmission of new pathogens. The scientific community should be prepared not just to respond to emerging infections, but also to anticipate their emergence. This is where monoclonal antibody research can help. A powerful monoclonal antibody that cross-reacts with numerous coronaviruses, for example, would be extremely valuable in averting future epidemics. As a result, monoclonal antibodies are expected to remain effective weapons in the fight against infectious illnesses.