
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Since vaccination does not offer 100% protection against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the demand for antiviral preparations enhancing the immune defense for preventing SARS-CoV-2 infections remains high. Two previous studies have provided crucial evidence on the effectiveness of Echinacea preparations against viral infections, including SARS-CoV-2.
Although its inhibiting effects do not always reach statistical significance for a particular virus, Echinacea purpurea (L.) shows antiviral activity against several enveloped viruses, including SARS-CoV-2 in both in vitro and in vivo physiological concentrations.
About the study
In the current study, the researchers reviewed a total of 1,687 articles from PubMed and EMBASE databases. From these, two studies met the eligibility criteria and were, therefore, selected for the systematic review. During the initial screening, 988 articles were excluded and 58 more were further excluded, as they did not report respiratory tract or coronavirus infections.
The researchers only qualitatively described outcomes from the two referenced studies. The first study was a double-blinded, placebo-controlled, monocentric randomized controlled trial (RCT) published in 2012, whereas the second study was a double-blinded multi-center RCT was published in 2021 and discussed the use of an Echinacea extract formulated in tablets that were given to children between the ages of 4-12 years living in central Switzerland.
In the selected studies, nasopharyngeal samples for virus testing were collected, blinded, randomized, and adequately controlled. A total of almost 1,000 patient data set was referenced, which included patients who were prescribed 65% (v/v) ethanolic extract (tincture or tablet form) of Echinacea purpurea for four months.
Study findings
Both studies demonstrated the preventive antiviral effects of Echinacea by varying parameters. Taken together, these studies screened numerous respiratory pathogens, some of which included influenza A H1/H3, influenza B, respiratory syncytial virus, and coronavirus 229E/OC43/NL63/HKU1.
Overall, 755 subjects were randomized to receive E. purpurea and 717 participants were eligible for analysis as per safety collective (SAF) in the control (placebo) group of the first referenced study. From 355 eligible subjects of the Echinacea group, there were 21 coronavirus infections and the incidence rate was 5.9%.
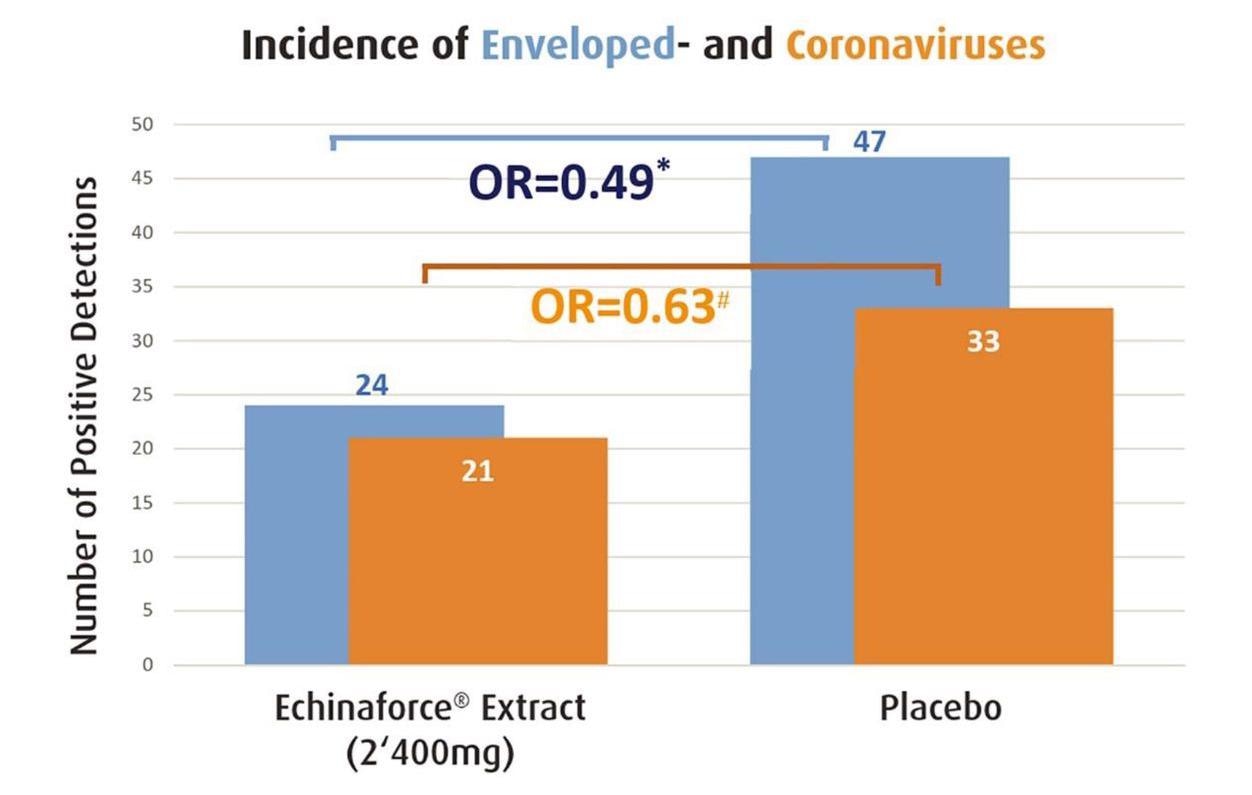
Echinacea significantly prevented enveloped virus infections (blue bar, * p=0.0114) and thereof coronaviruses 229E, HKU, NL63 and OC43 (orange bar, ♯ p>0.05) as measured by Jawad et al. 2012. Prevention of coronaviruses was significant for the ITT sub-group (p=0.010).
From the 362 eligible subjects of the control group, coronavirus infections totaled 33 with an incidence rate of 9.1%. Preventive effects on coronaviruses were statistically significant in participants in both the Echinacea and placebo groups who actively utilized their diary to report adverse events and cold-related symptoms. The findings indicated an overall incidence rate of 5.5% for Echinacea and 14.6 % for placebo.
The second referenced study determined Ct values for respective incidence tests, wherein the authors estimated the number of ribonucleic acid (RNA) copies as a measure for virus concentration in the nasopharyngeal sample. Overall, 57 and 72 samples were positively tested for messenger RNA (mRNA) from any respiratory virus during prevention with Echinacea and control, respectively.
A higher Ct value indicates a lower viral load represented by the sample’s RNA level. Echinacea significantly increased the average Ct value by 6.1 Ct units, from 25.0 to 31.1, thus resulting in a 98.5% reduction in coronavirus concentration relative to Vitamin C.
Overall, four months of supplementation with Echinacea reduced the virus concentration in nasopharynx swab samples and the overall incidence of SARS-CoV-2 in adults and children. In the 2012 study, under the influence of Echinacea, the viral numbers were reduced to below detection limits, whereas the 2021 study results showed lower Ct values. Therefore, the incidences with Echinacea and Ct values are complementary parameters indicative of the antiviral effects of Echinacea.
A clinical study carried out from November 2020 until May 2021 was also included in this review to confirm the applicability of the antiviral benefits of Echinacea. This clinical study investigated the effect of an Echinacea purpurea preparation in dosages within the range of 2,400 mg to 4,000 mg extract per day in 120 adults over five months.
In the Echinacea and control groups, five and 14 samples tested positive for SARS-CoV-2. During acute SARS-CoV-2 episodes, Echinacea treatment significantly reduced the overall virus load by about 99%, the time to virus clearance by 4.8 days, and fever days to 1 day versus 11 days as compared to the control group.
Conclusions
As hypothesized, the review findings confirm the general preventive effects of Echinacea purpurea extracts against coronavirus infections in both adults and children. While Echinacea reduced symptom development, significantly reduced their viral loads, and shortened the duration of illness in children, there is unpublished clinical evidence that further demonstrates its efficacy in adults as well. The clinical findings of the in vitro experiments further complement these findings.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Simon, N., Karin, A. W., Rainer, S., et al. (2021). Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children. medRxiv. doi:10.1101/2021.12.23.21267893. https://www.medrxiv.org/content/10.1101/2021.12.23.21267893v1
- Peer reviewed and published scientific report.
Nicolussi, Simon, Karin Ardjomand-Woelkart, Rainer Stange, Giuseppe Gancitano, Peter Klein, and Mercedes Ogal. 2022. “Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children.” Microorganisms 10 (2): 211. https://doi.org/10.3390/microorganisms10020211. https://www.mdpi.com/2076-2607/10/2/211.