
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Significance of the Omicron variant
In November 2021, the SARS-CoV-2 Omicron (B.1.1.529) variant was designated as a variant of concern (VOC) by the World Health Organization (WHO). This was largely due to the numerous mutations in the Omicron’s spike (S) protein region, many of which have previously been found to reduce both vaccine-induced and previous COVID-19-associated immunity.
Various animal models were rapidly developed for the preclinical studies of COVID-19-associated therapeutics and vaccines. As a result, the Golden Syrian hamster model has become well established. It exhibits clinical symptoms of severe COVID-19 disease compared to the ferret and non-human primate models representing asymptomatic and mild disease. Moreover, Syrian hamster models can effectively determine the efficacy of therapeutic interventions and can also be used for cross-protection studies after proper optimization.
About the study
In the present in vivo study conducted in Syrian hamster models, the pathogenicity and protection associated with convalescent immunity from a strain that is 99.99% similar to the SARS-CoV-2 wild-type strain against the Omicron variant were determined.
Apart from the B.1.1.529 variant isolated at the United Kingdom Health Security Agency (UKHSA), the prototype SARS-CoV-2 Australia/VIC01/2020 variant isolated in January 2020 provided by the Doherty Institute, Australia was used in the study. A dose-down experiment with lower doses of SARS-CoV-2 strains with homologous reinfection at an optimized timepoint in hamsters was performed to determine the impact of infection, waning immunity over time, and cross-protection against the Omicron variant.
The hamsters were infected intranasally with four different titers of the SARS-CoV-2 Australia/VIC01/2020 (VIC01) variant to achieve target doses. After 50 days of initial VIC01 infection, three hamsters from each convalescent group were rechallenged with either Omicron or VIC01 variants, thus resulting in 12 hamsters rechallenged with VIC01 and Omicron, respectively.
There were two age-matched naïve hamster control groups in the study, and about six and 11 hamsters in these control groups were infected with VIC01 and Omicron, respectively.
Back titration of infected stocks by focus forming assay (FFA) ensured that comparable doses of Omicron and VIC01 variants were administered to the control and rechallenged groups. Additionally, the longitudinal humoral immunity was assessed in hamsters on days 20 and 41 post-challenge.
Study findings
The experiments demonstrated high neutralizing antibody titers at days 20 and 41 post-challenge, irrespective of the dose of variants administered to the hamsters. Similarly, the rechallenge performed at 50 days post-challenge showed a high level of circulating antibodies. However, the groups of hamsters that received the VIC01 target dose of 5E+02 had lower circulating neutralizing antibodies as compared to two other higher target dose groups of 5E+04 and 5E+03.
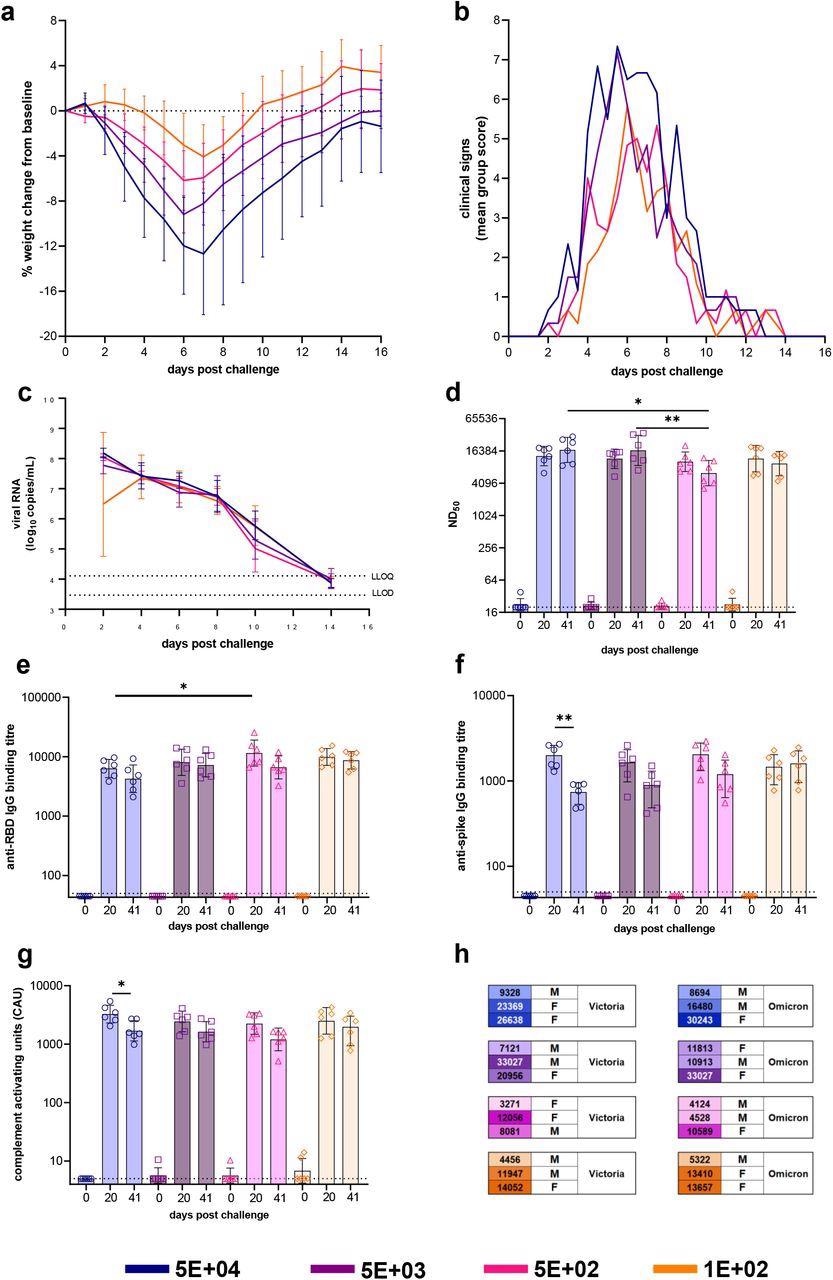
Dose-Ranging with VIC01. Hamsters were monitored for (a) weight change (lines represent group means, error bars represent standard deviation) and (b) clinical signs (lines represent group means) following challenge with VIC01. Hamsters challenged with 5E+04 lost significantly more weight overall (area under curve) than hamsters challenged with 5E+02 (P=0.0146) and 1E+02 (P=0.0058). Throat swabs were collected at days 2, 4, 6, 8 10 and 14 for all virus-challenged groups. (c) Total viral RNA was quantified by RT-qPCR at all sample time points. Lines show group geometric means, error bars represent standard deviation. The dashed horizontal lines show the lower limit of quantification (LLOQ) and the lower limit of detection (LLOD). Hamsters underwent gingival bleeds for sera at baseline, 20 and 41 days post-challenge for assessment of the humoral response. (d) Neutralizing antibody titers, (e) SARS-CoV-2 RBD-specific IgG binding antibodies, (f) SARS-CoV-2 spike-specific IgG binding antibodies and (g) SARS-CoV-2 spike-specific antibody-dependent complement deposition were assessed in challenged hamsters at baseline, day 20 and day 41 post-challenge. Bars represent group means and error bars represent standard deviation. All statistical analysis between groups was carried out using one-way ANOVA with Tukey’s correction. The dashed horizontal lines represent the lower limit of quantification (LLOQ) of the assays. (h) At 50 days post-challenge hamsters, each dose group was split into three and re-challenged with either VIC01 or Omicron. Numbers neutralizing antibodies (ND50) titers at day 41 post-challenge. Males and females were split equally between the VIC01 and Omicron re-challenge groups. Groups are identified according to their ‘target’ dose.
By day 20, the SARS-CoV-2 receptor-binding domains (RBD)-specific immunoglobulin G (IgG) binding antibodies in sera showed an increasing trend from baseline for all infected hamster groups. On the 20th day, the RBD-specific IgG binding antibodies titers in 5E+04 groups were significantly lower as compared to the antibodies titers in 5E+02 groups.
Around day 41 post-challenge, all of the challenged groups showed a decline in RBD-specific IgG binding antibodies titers. The hamster group with a target dose of 5E+04 showed a significant decline in antibody titers from day 20 to 41.
The naïve hamster control groups parallelly challenged with either Omicron or VIC01 variant demonstrated that the Syrian hamsters are susceptible to experimental infection with the SARS-CoV-2 Omicron variant. However, the infection with a high dose of Omicron variant in hamsters produced fewer clinical signs and lesser weight loss as compared to the SARS-CoV-2 ancestral variant at a comparable dose. On the contrary, the viral shedding in the upper respiratory tract was similar in the VIC01 and Omicron infected hamsters.
The reinfection of Syrian hamsters with either the Omicron or VIC01 variant does not induce significant clinical symptoms of COVID-19 in hamsters previously infected with the SARS-CoV-2 ancestral variant.
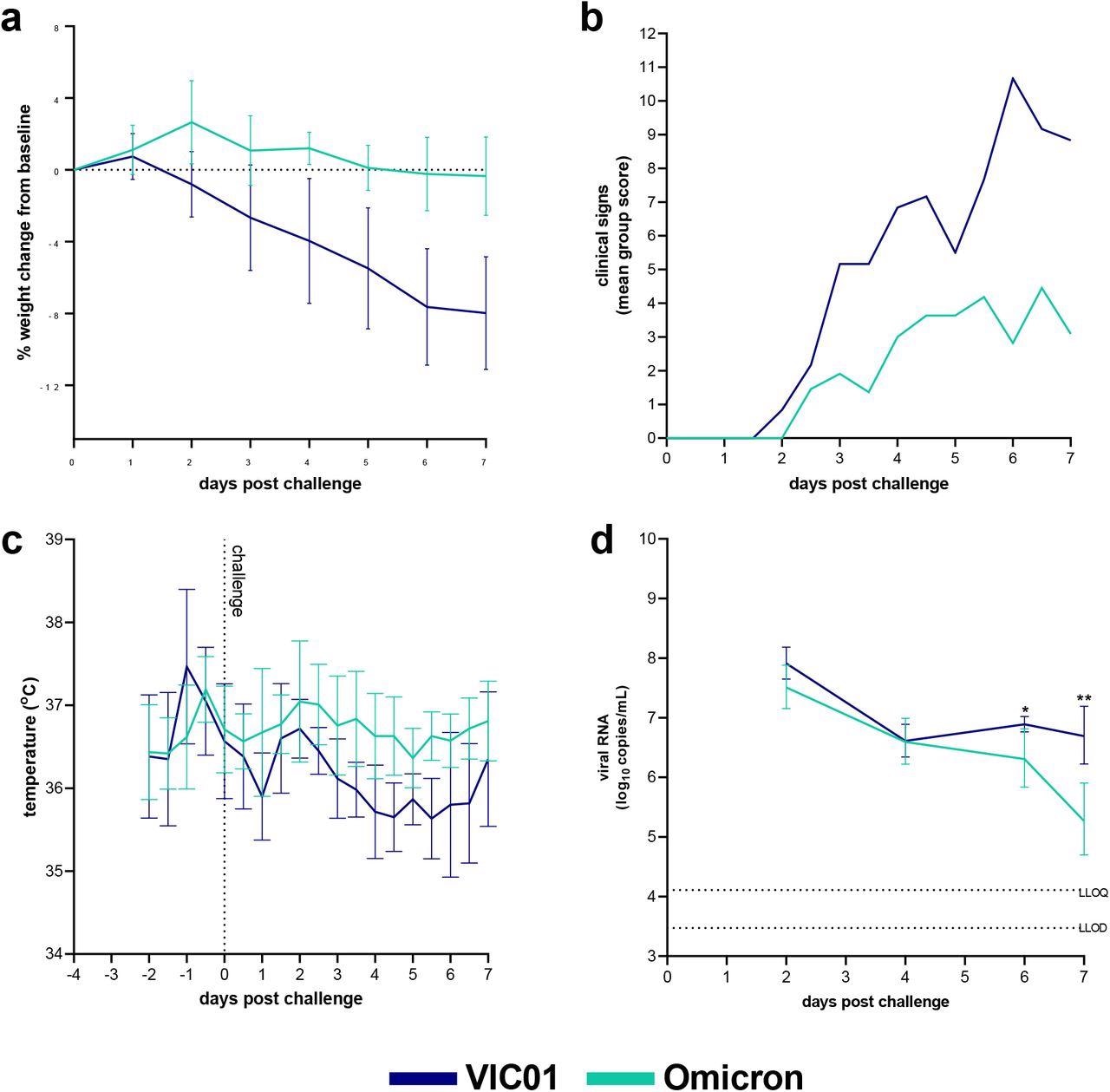
Omicron and VIC01 Challenge in naïve hamsters. Hamsters were monitored for (a) weight change, (b) clinical signs and (c) temperature following challenge with VIC01 or the Omicron variant. Hamsters challenged with VIC01 experienced significantly more total weight loss (area under the curve, P=0.0007) than hamsters challenged with Omicron. Throat swabs were collected at days 2, 4, 6 and 7 for all virus-challenged groups. (d) Viral RNA was quantified by RT-qPCR at all sample time points. Lines show group geometric means, error bars represent standard deviation. The dashed horizontal lines show the lower limit of quantification (LLOQ) and the lower limit of detection (LLOD). At day 6 and day 7 Omicron challenged hamsters shed significantly less virus (P=0.0136 and P=0.0012 respectively) than hamsters infected with VIC01. There was no significant difference between total amount of viral RNA shed.
Conclusions
The study results established that Omicron infection in naïve Syrian hamsters resulted in less severe COVID-19 as compared to the SARS-CoV-2 Australia/VIC01/2020 variant. Similarly, the clinical symptoms of COVID-19 were nearly absent in convalescent hamsters reinfected with the Omicron variant 50 days post-initial infection with SARS-CoV-2 Australia/VIC01/2020 variant. A strong cellular immune response was associated with reinfection due to the presence of high circulating antibodies at 50 days post-reinfection.
Overall, the current study's findings indicated that the immunity from the wild-type SARS-CoV-2 infection protects against the Omicron variant in the hamster model. However, further studies are required to conclude if Omicron is less pathogenic in Syrian hamsters and explore the effects of RBD-associated Omicron mutations in humans.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ryan, K. A., Watson, R. J., Bewley, K. R., et al. (2021). Convalescence from prototype SARS-CoV-2 protects Syrian hamsters from disease caused by the Omicron variant. bioRxiv. doi:10.1101/2021.12.24.474081.https://www.biorxiv.org/content/10.1101/2021.12.24.474081v1.
- Peer reviewed and published scientific report.
Ryan, Kathryn A., Kevin R. Bewley, Robert J. Watson, Christopher Burton, Oliver Carnell, Breeze E. Cavell, Amy Challis, et al. 2023. “Syrian Hamster Convalescence from Prototype SARS-CoV-2 Confers Measurable Protection against the Attenuated Disease Caused by the Omicron Variant.” Edited by Jacob S. Yount. PLOS Pathogens 19 (4): e1011293. https://doi.org/10.1371/journal.ppat.1011293. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1011293.