The Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) coronavirus disease 2019 (COVID-19) vaccine was one of the first coronavirus disease 2019 (COVID-19) vaccines that were provided emergency-use approval (EUA) in the United States after its efficacy was proven in multiple clinical trials.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Real-world data on BNT162b2 have added to the credibility of the vaccine in reducing laboratory-confirmed and community-acquired severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, viral loads, disease severity, hospitalizations, and deaths after completion of the double dosing regimen.
However, multiple reports of breakthrough infections in fully vaccinated persons have been reported, particularly following the emergence of the SARS-CoV-2 Omicron variant. Healthcare workers, especially those caring for patients with COVID-19, are at an exceptionally high risk of infection owing to prolonged and repeated exposure.
Previous studies have reported the occurrence of SARS-CoV-2 breakthrough infections in fully vaccinated individuals to be correlated with neutralizing antibody titers formed as a result of vaccination during the peri-infection period. These studies have also reported that patients’ humoral responses were substantially decreased six months after receiving the second dose of the BNT162b2 vaccine.
In a recent study published on the preprint server medRxiv*, researchers evaluate the effects of a third (booster) dose of the BNT162b2 mRNA vaccine on antibody titers and breakthrough infections in Japanese healthcare workers.
About the study
In the current study, researchers investigated the transition of anti-SARS-CoV-2 receptor-binding domain (RBD) immunoglobulin G (IgG) and neutralizing antibody titers in 41 healthcare workers at Nara Medical University in Japan. These study participants had received the double-dose Pfizer-BNT162b2 vaccine between March 10, 2021, and March 30, 2021.
Study participants provided peripheral blood samples a total of seven times for serology assays. The first sample was collected before receiving the first dose of the vaccine, the second after one week after receiving the first vaccine, then in an interval of one week, one month, three months, and six months after receiving the second vaccine. All eligible patients were over 20 years of age and had no history of suspected clinical SARS-CoV-2 infection.
Study findings
Serology analysis revealed that anti-SARS-CoV-2 RBD IgG levels were slightly increased seven days after the first dose of the Pfizer-BNT162b2 vaccine to an average of 43.8 binding antibody units (BAU) /ml as compared with those before the vaccination at 10.3 BAU/ml. The highest titers were observed seven days after the second dose of the Pfizer-BNT162b2 vaccine at 4,549 BAU/ml.
Antibody titers reduced by factors of 3.4 after one month (1,341 BAU/ml), 6.6 after three months (684.2 BAU/ml), and 15.3 after six months (297.3 BAU/ml) then declined over time and reduced to less than 10% at six months after the second dose. Workers with low anti-SARS-CoV-2 RBD IgG levels also had low neutralizing antibody titers.
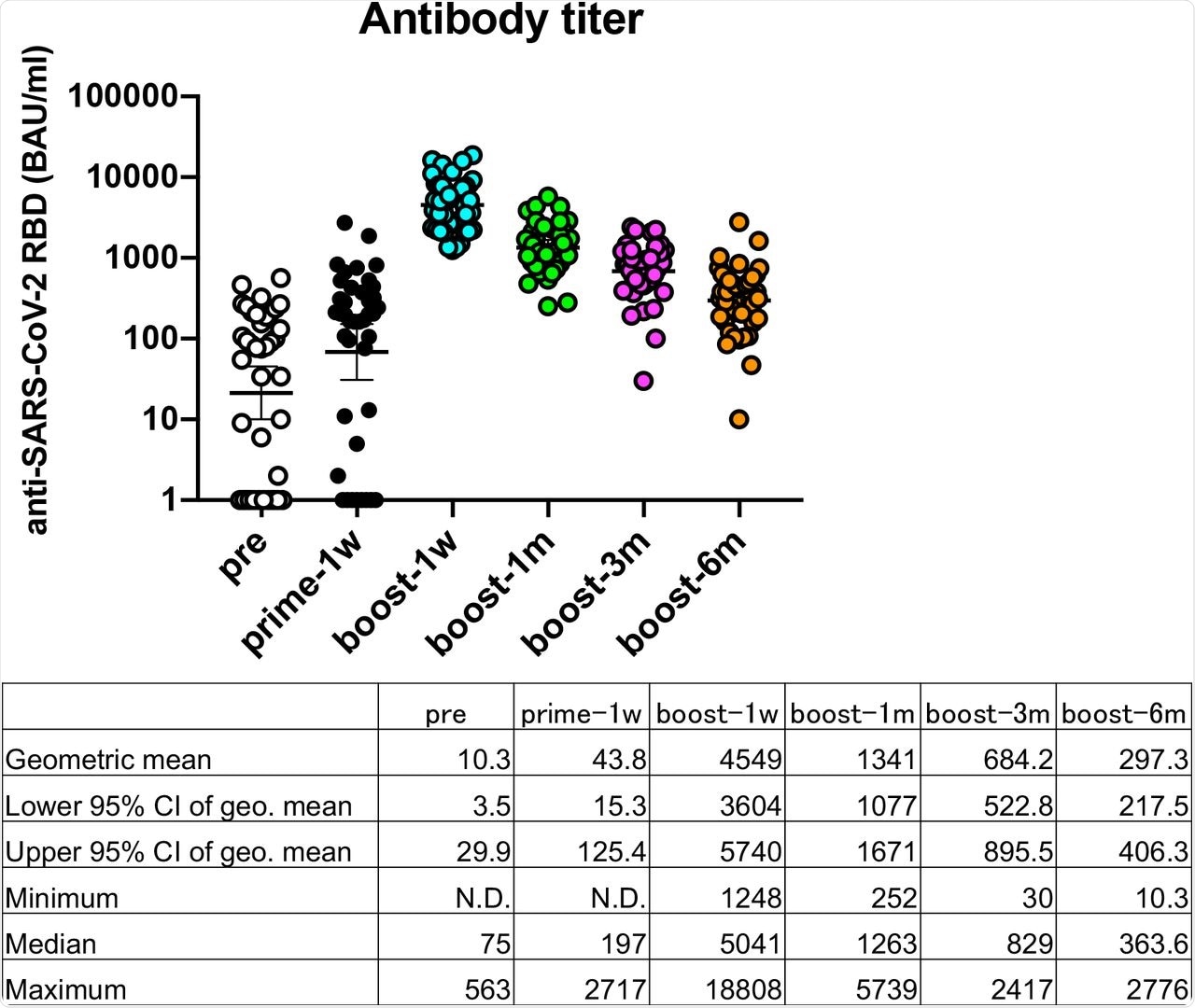
Transition of anti-SARS-CoV-2 responses after the COVID-19 mRNA BNT162b2/Pfizer vaccination
Implications
The current study reveals the importance of booster vaccine doses for healthcare workers, especially those with low anti-SARS-CoV-2 RBD IgG levels. These results also encourage further research on adjusting current COVID-19 vaccine strategies to increase the duration of anti-spike antibody titers in blood for the general public through booster shots.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kitabatake, M., Ouji-Sageshima, N., Sonobe S, et al. (2021) Transition of antibody titers after the SARS-CoV-2 mRNA vaccine in Japanese healthcare workers. medRxiv. doi:10.1101/2021.12.28.21268435. https://www.medrxiv.org/content/10.1101/2021.12.28.21268435v1.
- Peer reviewed and published scientific report.
Kitabatake, Masahiro, Noriko Ouji-Sageshima, Shota Sonobe, Ryutaro Furukawa, Makiko Konda, Atsushi Hara, Hiroyasu Aoki, et al. 2023. “Transition of Antibody Titers after SARS-CoV-2 MRNA Vaccination in Japanese Healthcare Workers.” Japanese Journal of Infectious Diseases 76 (1): 72–76. https://doi.org/10.7883/yoken.JJID.2022.041. https://www.jstage.jst.go.jp/article/yoken/76/1/76_JJID.2022.041/_article.