Currently, monoclonal antibodies (mAbs) are being used as the first-line therapy in the management of mild-to-moderate coronavirus disease 2019 (COVID-19) among high-risk patients. These mAbs accelerate the decay of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in the upper respiratory tract. However, the effects of these agents on the duration of shedding viable virus remains unknown.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Bamlanivimab, which is one type of clinically used neutralizing mAb, binds to the spike protein of SARS-CoV-2 and prevents the viral uptake into host cells. Bamlanivimab, along with Etesevimab, has been granted emergency use authorization by the United States Food and Drug Administration (FDA) for use for post-exposure prophylaxis and in high-risk, non-hospitalized SARS-CoV-2 patients.
A recent study published on the medRxiv* preprint server discusses the viral culture analysis of 54 participants enrolled in the ACTIV-2 randomized placebo-controlled trial of bamvalinimab monotherapy for non-hospitalized adults with mild to moderate COVID-19. The present study was based on the rationale that fully understanding the potential benefits of mAbs and other treatments may aid in determining their optimal use for preventing and treating SARS-CoV-2 infection.
About the study
The present study aimed to analyze the effect of Bamlanivimab monotherapy in adults suffering from mild-to-moderate COVID-19 who did not require hospitalization.
Herein, viable viral shedding was compared to changes in anterior nasal sample SARS CoV-2 ribonucleic acid (RNA) over time after treatment with mAb. Viruses were cultured from anterior nasal swabs samples from participants enrolled in the ACTIV-2 study. Participants had a baseline viral load of at least 6.0 log10 SARS-CoV-2 RNA copies/mL from available swab samples that were collected on study days zero, one, two, three, and seven.
A total of 69 out of the 317 participants in the ACTIV-2 study met the inclusion criteria for the primary analysis. Of these, 310 had available baseline anterior nasal swabs, 94 had baseline viral load of less than or equal to 6.0 log10 SARS-CoV-2 RNA copies/mL, and 73 had swabs available at days 0, 1, 2, 3, and 7.
On day zero, 39 patients received placebo and 30 received Bamlanivimab therapy, 20 of which were given the 7,000 mg dose and ten received the 700 mg dose. The viral culture was studied on days one, two, three, and seven. Assessment of shedding of the culturable virus was performed on days zero, one, and two. If found positive, the culture was further studied on days three and seven.
Study findings
Baseline anterior nasal viral load and viral culturability were similar between the groups. Culture positive baseline samples were obtained from 100% participants in the placebo arm and 93% samples in the mAb arm.
On day one, nasal swab viral loads were similar between the two groups, while a significant difference in culture positivity was detected in 41% in the placebo arm and 0% in the bamlanivimab treatment group.
In the placebo group, the lowest viral load associated with a positive culture was 5.5 log10 RNA copies/mL, 64% of which were also found to be culture positive. Comparatively, all samples from the bamlanivimab cohort with equivalent viral loads were culture negative.
On day two, 18% of participants in the placebo arm remained culture positive, while all participants in the bamlanivimab arm were culture negative. Additional testing of samples found that five of the 15 tested placebo participants were culture positive on day three and one of 15 tested placebo participants remained culture positive on day seven.
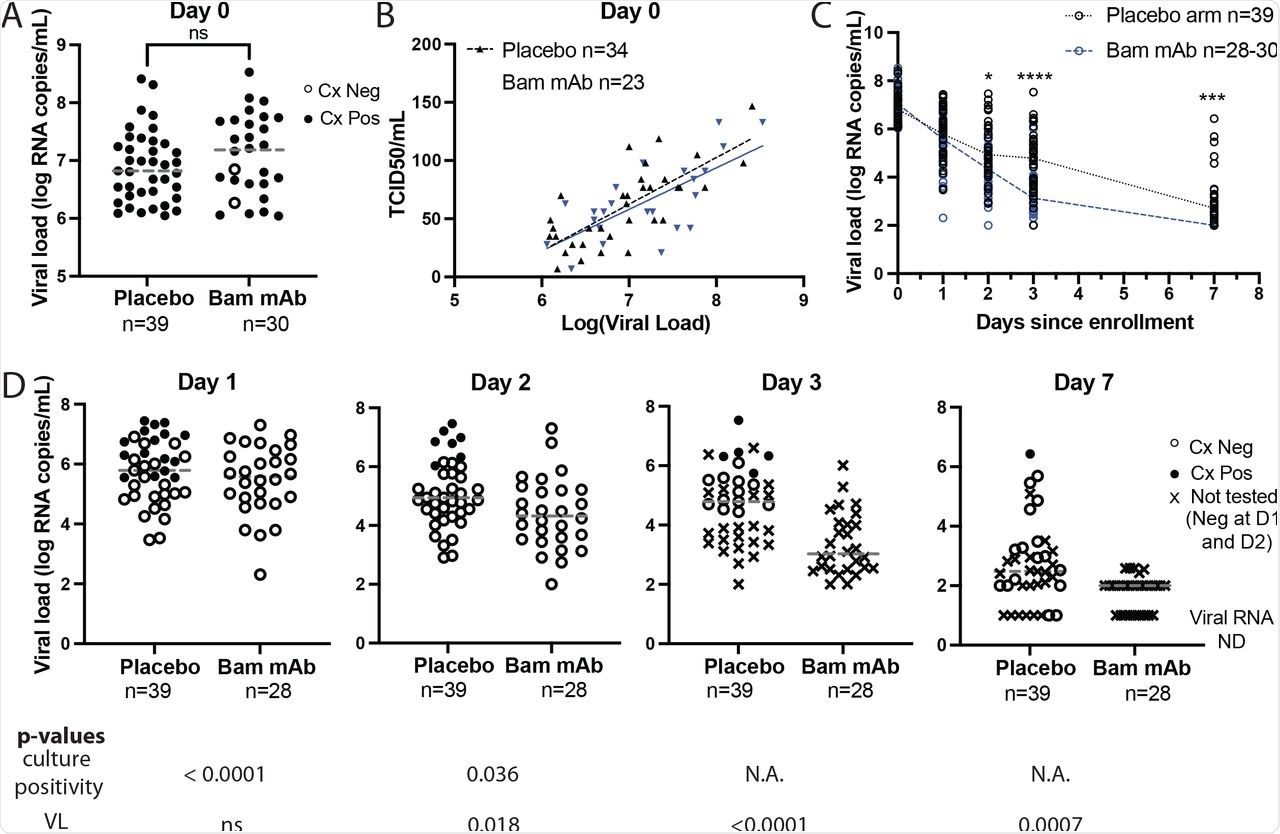 Bamlanivimab treatment results in rapid SARS-CoV-2 culture conversion.
Bamlanivimab treatment results in rapid SARS-CoV-2 culture conversion.
In addition, four participants with treatment-emergent bamlanivimab resistance mutations were evaluated in a substudy to test the hypothesis that early viral culture clearance gained through bamlanivimab treatment was due to mAb binding and neutralization of virions. The researchers were also interested in determining whether mAb resistance would lead to renewed shedding of the culturable virus.
Samples from three participants were found to contain the emergent E484K mutation and one had the emergent E484Q mutation. These subjects rendered positive viral cultures at day zero, all of which turned negative by day one.
However, the emergence of the E484K mutation after bamlanivimab treatment was associated with a rebound in viral loads and positive viral cultures with rising Median Tissue Culture Infectious Dose (TCID50) levels. Meanwhile, the E484Q mutation conferred only modest increases in viral load and no re-emergence of positive viral cultures.
Conclusions
The results of the study reflected the effectiveness of bamlanivimab treatment in rapidly reducing the shedding of culturable viruses from the anterior nasal region. This is an indication of the reduction in the viral load in the patient.
Further, the current study underlines the importance of viral culture assays for evaluating the effectiveness of mAb therapies. This would help in earlier detection of the efficacy of mAbs in clinical trials.
The study findings also suggest that the use of mAbs in the treatment of COVID-19 patients reduces the risk of secondary transmission by reducing the period of infectiousness in an individual. However, the emergence of resistant variants can cause the return of the infection and its transmission.
Additionally, the results emphasized the use of combination therapies to prevent viral mutations and the subsequent emergence of resistant strains. The authors concluded that understanding the results of any therapeutic intervention on SARS-CoV-2 is very crucial. To this end, this data affects the development of any treatment plan and recommendations given to the patient after the treatment is completed.
Taken together, mAb treatment for COVID-19 has the potential to confer a potential public health benefit in reducing the period of infectiousness, as well as the risk of secondary transmission.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Boucau, J., Chew, K., Choudhary, M., et al. (2021). Monoclonal antibody treatment drives rapid culture conversion in SARS-CoV-2 infection. medRxiv. doi:10.1101/2021.12.25.21268211. https://www.medrxiv.org/content/10.1101/2021.12.25.21268211v1.
- Peer reviewed and published scientific report.
Boucau, Julie, Kara W. Chew, Manish C. Choudhary, Rinki Deo, James Regan, James P. Flynn, Charles R. Crain, et al. 2022. “Monoclonal Antibody Treatment Drives Rapid Culture Conversion in SARS-CoV-2 Infection.” Cell Reports Medicine 3 (7): 100678. https://doi.org/10.1016/j.xcrm.2022.100678. https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(22)00214-2.