The emerging Omicron variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has raised serious concerns about the course of the ongoing coronavirus disease 2019 (COVID-19). With almost 40 mutations in the spike protein of the virus, this variant has shown a high capacity to evade the antibodies elicited earlier and spread more rapidly and extensively than any earlier variant.
For this reason, Omicron was quickly designated a variant of concern (VOC) by the World Health Organization (WHO). A recent study showed that vaccine recipients who had received any of the three approved COVID-19 vaccines in the USA failed to show protection against this VOC. The case with protection against reinfection was similar. An additional dose was, however, strongly protective, producing higher neutralizing antibody (nAb) levels as well as greater breadth of neutralization.
This study was followed up recently with the evaluation of additional booster dose efficacy in patients who were currently being treated for cancer and recognized as being immunocompromised. This group has shown reduced responsiveness to the vaccine, which is partly overcome by an additional dose. A new preprint research paper posted to the medRxiv* server reports the induction of high neutralizing activity in these patients against the Omicron VOC with a booster dose.

Study: COVID-19 mRNA Booster Vaccines Elicit Strong Protection Against SARS-CoV-2 Omicron Variant in Patients with Cancer. Image Credit: haidaralf/Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What Did the Study Show?
The investigators used a very sensitive pseudovirus neutralization assay of their own design, comparing the nAb response in cancer patients against the Delta vs Omicron variants, as well as to the D614G variant that was prevalent during the early days of the pandemic.
The results showed that after two doses of a messenger ribonucleic acid (mRNA) vaccine, the neutralization titer against the Delta and Omicron VOCs was reduced by 4-fold and 21-fold, respectively, compared to D614G
While none of the patients failed to show detectable neutralizing titers against the D614G variant, response failure was seen in 13% and over 50% of assays with the Delta and Omicron variants. This shows that in cancer patients on active treatment, Omicron resists neutralization by antibodies elicited by a double dose of either of the mRNA vaccines.
With a booster third dose, the nAb titers zoomed up. Increased neutralization was observed with all variants, and Omicron neutralization was only 5-fold less compared to D614G. This is very similar to the ~3.5-fold lower neutralization of the Delta VOC after 3 doses, and the 4-fold less neutralizing activity for Delta vs. D614G after two doses.
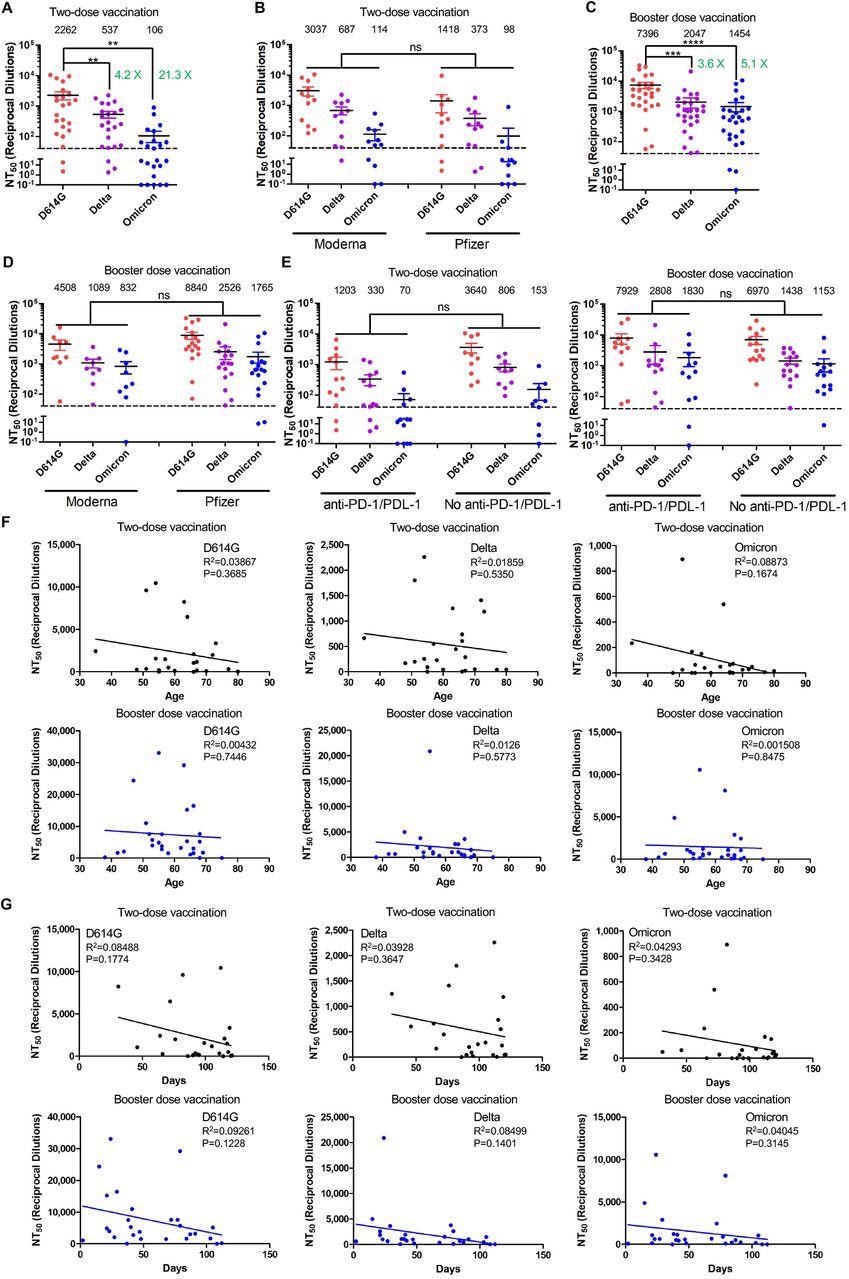
The Omicron variant exhibits strong immune-escape in two-dose-vaccinated patients with cancer which is overcome by booster vaccinations. Gaussia luciferase reporter gene-bearing lentiviral vector was pseudotyped with spike from SARS-CoV-2 variant of interest, and pseudotyped viruses were then incubated with serially-diluted patient serum before being used to infect HEK293T-ACE2 cells. Culture media were changed after overnight infection and were measured for the luciferase activity at 48 and 72 hrs post-infection. (A and B) Sera from 23 patients with solid cancer collected after the second mRNA vaccine dose (12 mRNA-1273 and 11 BNT16b2) were used to neutralize pseudotyped virus bearing the spike of D614G, Delta and Omicron variants, and resulting 50% neutralization titers (NT50) were calculated. (C and D) Sera from 27 patients with solid cancer collected after booster vaccination (9 mRNA-1273 and 18 BNT16b2) were used to neutralize pseudotyped viruses of D614G, Delta or Omicron variants, with NT50 calculated. (E) NT50 values against D614G, Delta and Omicron variants in cancer patients, with or without anti-PD-1/PDL-1 treatments: two-dose vaccination and booster dose vaccination. (F) Correlative analyses between NT50 values against variants and patient’s age: two-dose vaccination and booster vaccination. (G) Correlative analyses between NT50 against variants and sera collection days: two-dose vaccination and booster vaccination. In all cases, mean NT50 values are displayed at the top of each plot; bars represent mean +/-standard error, and significance is determined by one-way ANOVA with Bonferroni’s multiple testing correction. P-values are represented as ** p < 0.01, *** p < 0.001, **** p < 0.0001; ns, not significant (defined as P > 0.05).
After three doses, there were no non-responders against either D614G or Delta, while about a tenth of Omicron assays showed a failure to respond. This indicates that an additional dose extends the breadth and strength of neutralization against the Omicron VOC.
Some evidence of waning humoral immunity was obtained, but there was no correlation with age, perhaps because most patients were elderly and the sample size was small. Further studies will be required to confirm the positive impact of the third dose of vaccine in protecting cancer patients on treatment against Omicron infection.
The PD-1 protein found on T cells is involved in the cellular immune response to a host of antigens, including cancer cells. When PD-1 is bound to the PD-L1 protein, it inhibits T cells from killing cancer cells, among other cells.
The use of PD-1/PD-L1A blockers in patients with solid tumors was also examined as a possible risk factor for low neutralizing response to these vaccines. However, there was no difference in these patients compared with those who did not receive these drugs, either after two or three vaccine doses.
What Are the Implications?
“Our results demonstrate that patients with cancer who received booster dose of mRNA vaccine displayed a significantly greater neutralizing capacity against the Omicron variant in comparison to those recipients of two-doses of mRNA vaccine.”
These gratifying results corroborate the findings of earlier studies in healthcare workers. They indicate the probable efficacy of a booster dose strategy in preventing an Omicron-driven surge among cancer patients. The mechanism of such broad protection remains to be unraveled.
Clinical validation of these findings must be performed before deciding the degree of protection that may be conferred against breakthrough infection after a third mRNA vaccine dose. However, at present, it seems to be clear that cancer patients being treated for the disease are not, per se, resistant to the booster effects of an additional vaccine dose.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zeng, C. et al. (2021). COVID-19 mRNA Booster Vaccines Elicit Strong Protection Against SARS-CoV-2 Omicron Variant in Patients with Cancer. medRxiv preprint. doi: https://doi.org/10.1101/2021.12.28.21268398. https://www.medrxiv.org/content/10.1101/2021.12.28.21268398v1
- Peer reviewed and published scientific report.
Zeng, Cong, John P. Evans, Karthik Chakravarthy, Panke Qu, Sarah Reisinger, No-Joon Song, Mark P. Rubinstein, Peter G. Shields, Zihai Li, and Shan-Lu Liu. 2021. “COVID-19 MRNA Booster Vaccines Elicit Strong Protection against SARS-CoV-2 Omicron Variant in Patients with Cancer.” Cancer Cell, December. https://doi.org/10.1016/j.ccell.2021.12.014. https://www.cell.com/cancer-cell/fulltext/S1535-6108(21)00688-7.