
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The World Health Organization (WHO) recently designated the SARS-CoV-2 Omicron variant as a VOC due to the rapid surge of COVID-19 cases driven by this new strain and a large number of spike (S) protein-associated mutations in this variant.
Preliminary studies have demonstrated substantial reductions in neutralizing response towards Omicron within convalescent and vaccinated individuals. However, most of these studies had limited diversity in sample type, small sample size, or only compared Omicron with either the wild-type (WT) or a few other VOCs.
About the study
In the present study, the researchers compared the ACE2-binding inhibition, immunoglobulin G (IgG) binding, and antibody binding dynamics of the Omicron variant to all other SARS-CoV-2 VOCs and VOIs using a large sample of previously infected, vaccinated, and infected then vaccinated individuals.
The vaccinated and convalescent sera samples of adults and children from the first, second, and third COVID-19 waves in Germany were collected for the study. The convalescent sera samples with the SARS-CoV-2 WT, Alpha, and Delta variants were used for the current study.
Additional samples were collected from recipients of two homologous doses of mRNA-1273, AZD1222, or BNT162b2 vaccine, two heterologous doses of either AZD1222 and BNT162b2 or mRNA-1273 vaccine, three doses of BNT162b2 vaccine, as well as previously infected individuals who had received a single dose of BNT162b2 vaccine. Commercially available pre-pandemic sera samples were also used as controls for the unvaccinated groups.
IgG binding of the SARS-CoV-2 variants was determined using a previously published SARS-CoV-2 multiplex immunoassay, MULTICOV-AB. Next, the ACE2-binding inhibition of all SARS-CoV-2 VOCs was analyzed using a multiplex ACE2-RBD inhibition assay. Subsequently, the binding kinetics of receptor-binding domain (RBD)-specific antibodies from convalescent and vaccinated sera samples were analyzed using biolayer interferometry (BLI) analysis.
The effect of vaccine type and the number of doses on binding responses against Omicron were evaluated at one to two months and five to six months post-second dose in homologous vaccine recipients.
Next, binding responses against Omicron were compared between the BNT162b2 booster vaccinated, two-dose vaccinated, as well as convalescent individuals. Binding responses against Omicron between convalescent children and adult samples were also compared.
Study findings
IgG-binding capacity and ACE2-binding inhibition towards Omicron elicited by vaccination or previous infection were found to be significantly lower as compared to other variants. Moreover, most samples tested for ACE2-binding inhibition were non-responsive towards Omicron.
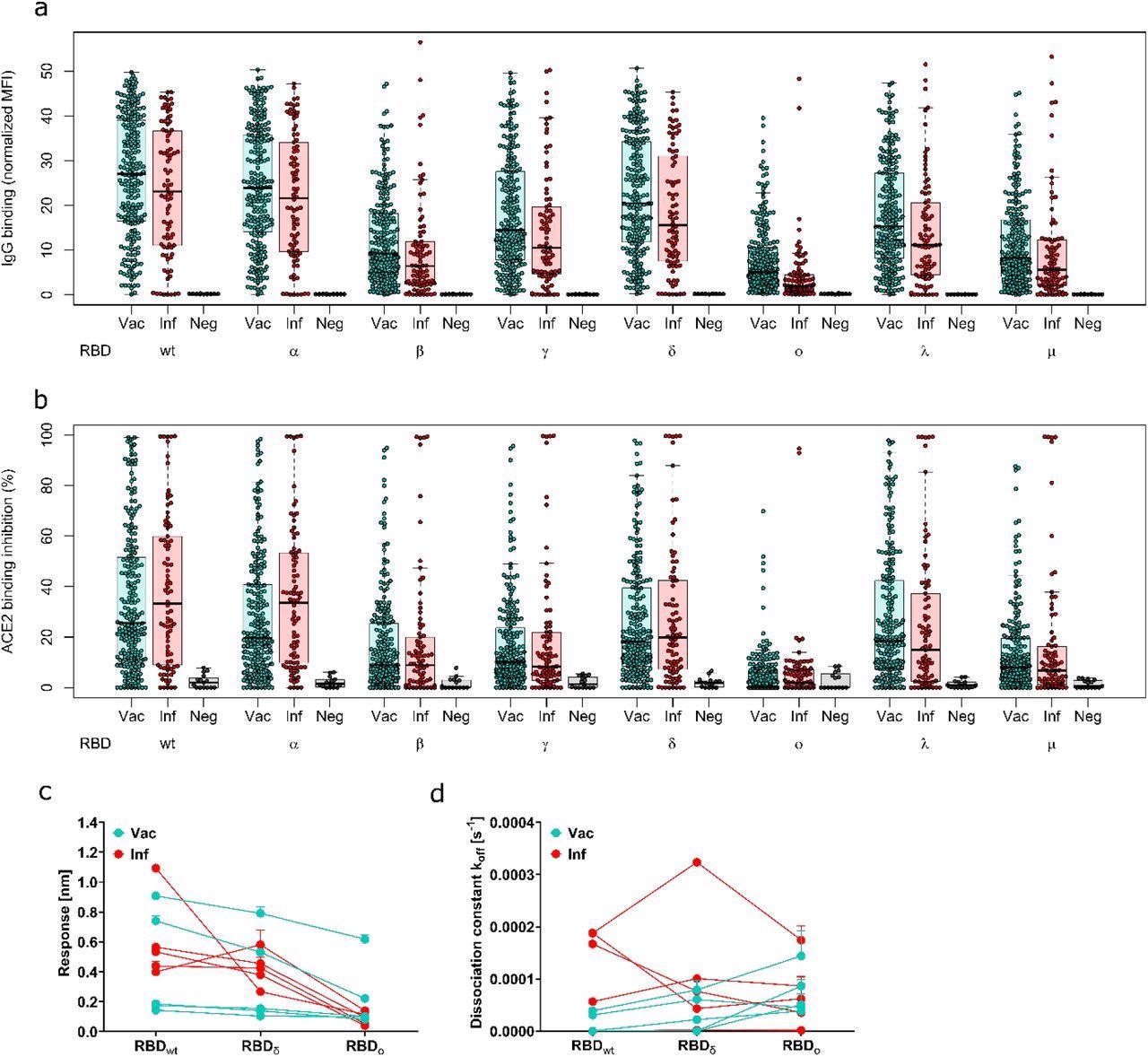
Antibody binding response is significantly reduced for Omicron compared to all other variants of concern. Binding response by pre-existing antibodies generated through either infection or vaccination was measured with MULTICOV-AB (a), RBDCoV-ACE2 (b) and Biolayer interferometry (c and d). (a) Boxplot showing that IgG binding is significantly reduced for omicron compared to all other variants of concern for both infected (n=86) and vaccinated samples (n=226). Negative samples are included as controls (n=15). (b) Boxplot showing that ACE2 binding inhibition is significantly reduced for Omicron compared to all other variants of concern for both infected and vaccinated samples. Boxes represent the median, 25th and 75th percentiles, whiskers show the largest and smallest non-outlier values. Outliers were determined by 1.5 IQR. (c and d) Binding kinetics of RBD specific antibodies from serum samples of convalescent and vaccinated individuals (both n=5). Binding response (c) and dissociation constant (d) were determined by 1:1 fitting model of the individual serum samples between the different RBD variants.
Similarly, the binding kinetics of RBD-specific antibodies indicated that the IgG-binding response and ACE2-binding inhibition towards the Omicron RBD were less than the WT and Delta strains of SARS-CoV-2. However, the IgG-binding response towards Omicron was more intact than the ACE2-binding inhibition.
In the one to two months post-vaccinated samples, convalescent vaccinated participants had the highest IgG-binding response towards the Omicron variant, followed by two-dose mRNA-1273 and AZD1222 vaccine recipients.
In the five to six months post-vaccinated samples, the IgG-binding capacity towards Omicron was lower in all participants, regardless of the vaccine received. However, those who received the two-dose AZD1222 vaccine exhibited a lower IgG-binding response as compared to the recipients of the homologous two-dose mRNA-1273 and BNT162b2 vaccines, or heterologous vaccination.
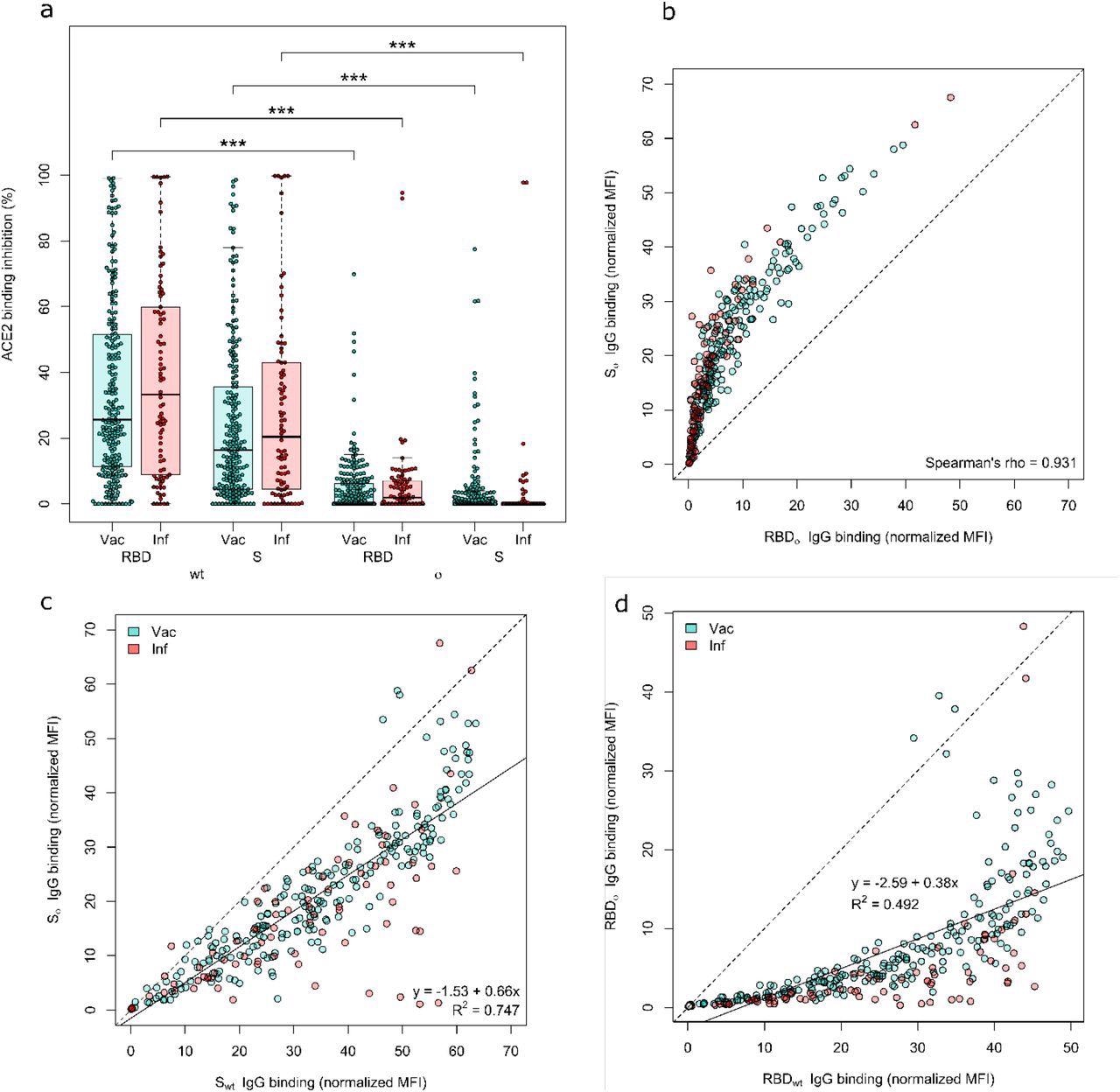
Both the Omicron RBD and Spike protein have minimal neutralizing activity. ACE2 binding inhibition (a) and IgG binding capacity (b-d) were compared for the Omicron RBD and S to the wild-type RBD and S. (a) Boxplot showing that ACE2 binding inhibition while significantly reduced for Omicron in general, is significantly reduced for the RBD compared to the Spike for both Vaccinated (n-=226) and infected (n=86) samples. Boxes represent the median, 25th and 75th percentiles, whiskers show the largest and smallest non-outlier values. Outliers were determined by 1.5 IQR. (b) Correlation analysis of IgG binding capacity for the Omicron spike compared to the Omicron RBD. Spearman’s rank was calculated to assess ordinal association between the variables. (c) Linear regression of IgG binding capacity for the Omicron Spike compared to wild-type Spike. (d) Linear regression of IgG binding capacity for the Omicron RBD compared to wild-type RBD. Statistical significance was calculated by Mann-Whitney U with *** indicating a p-value <0.001.
After receiving the third booster dose of the BNT162b2 vaccine, there was an increase in IgG-binding capacity and ACE2-binding inhibition towards all SARS-CoV-2 variants, including Omicron.
No difference in ACE2-binding inhibition was observed towards the Omicron variant among convalescent individuals with Alpha or WT infections. Comparatively, few convalescent Delta samples showed a higher ACE2-binding inhibition towards Omicron than the WT or Alpha strains.
The ACE2-binding inhibition towards Omicron was similar between children and adults. However, the antibody binding capacity was significantly lower in children than adults.
Conclusions
In the current study, the researchers found that the SARS-CoV-2 Omicron variant can still bind to ACE2, while pre-existing antibodies bind Omicron. Furthermore, the extent of RBD-associated mutations in Omicron inhibits the development of a neutralizing response.
The antibodies elicited by vaccination or previous infection had lower IgG-binding and ACE2-binding inhibition capacity towards the Omicron variant as compared to all other SARS-CoV-2 VOCs. This pronounced reduction in IgG-binding and ACE2-binding inhibition indicated the enhanced immune escape capacity of Omicron.
The study findings were in line with previous studies demonstrating the low neutralizing response against the SARS-CoV-2 Omicron variant in convalescent and vaccinated sera samples. However, the BNT162b2 booster vaccine provided a significant increase in antibody response against Omicron.
Taken together, the findings emphasized the need for continuous adaptation of vaccines against novel and highly contagious SARS-CoV-2 variants to eliminate COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Junker, D., Becker, M., Wagner, T. R., et al. (2021). Antibody binding and ACE2 binding inhibition is significantly reduced for the Omicron variant compared to all other variants of concern. medRxiv. doi:10.1101/2021.12.30.21267519. https://www.medrxiv.org/content/10.1101/2021.12.30.21267519v1.
- Peer reviewed and published scientific report.
Junker, Daniel, Matthias Becker, Teresa R Wagner, Philipp D Kaiser, Sandra Maier, Tanja M Grimm, Johanna Griesbaum, et al. 2022. “Antibody Binding and Angiotensin-Converting Enzyme 2 Binding Inhibition Is Significantly Reduced for Both the BA.1 and BA.2 Omicron Variants.” Clinical Infectious Diseases 76 (3): e240–49. https://doi.org/10.1093/cid/ciac498. https://academic.oup.com/cid/article/76/3/e240/6611494.