The ongoing coronavirus disease 2019 (COVID-19) pandemic has been caused by a novel coronavirus, namely, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This RNA virus causes mild to severe symptoms and has a high transmission rate. In the case of severe infection, patients often suffer a hyperinflammatory response, which has been associated with morbidity and mortality due to COVID-19 disease. Therefore, researchers believe that the early treatment of hyperinflammatory responses could decrease the rate of mortality. The LIVE-AIR phase 3 clinical trial is associated with early treatment of hyperinflammatory response with the humanized monoclonal antibody lenzilumab in COVID-19 patients with decreased oxygen saturation. The study results are posted to the medRxiv* preprint server.
 Study: Impact of Baseline Demographics and Risk Factors on Survival with Ventilation Using an Iterative Multivariate Logistic Regression Model. Twelve covariates were included in the model encompassing known risk factors for progression to IMV and/or death by Day 28: Baseline CRP (CRP), disease severity at randomization (severity), respiratory condition (asthma, COPD, interstitial lung disease), age >=65, diabetes (Type 1 or Type 2), lenzilumab (treated or placebo), BMI, time from admission to randomization, time from symptoms to randomization, heart condition (hypertension, coronary artery disease, congestive heart failure), male gender, vascular condition (cerebrovascular disorders, thrombosis or embolism), other risk factors (prior diagnosis of cancer; hematological or non-hematological), chronic kidney disease (including renal failure), or chronic liver disease (including hepatic failure). Statistical significance was reached for all features with a displayed p-value.. Image Credit: NIAID
Study: Impact of Baseline Demographics and Risk Factors on Survival with Ventilation Using an Iterative Multivariate Logistic Regression Model. Twelve covariates were included in the model encompassing known risk factors for progression to IMV and/or death by Day 28: Baseline CRP (CRP), disease severity at randomization (severity), respiratory condition (asthma, COPD, interstitial lung disease), age >=65, diabetes (Type 1 or Type 2), lenzilumab (treated or placebo), BMI, time from admission to randomization, time from symptoms to randomization, heart condition (hypertension, coronary artery disease, congestive heart failure), male gender, vascular condition (cerebrovascular disorders, thrombosis or embolism), other risk factors (prior diagnosis of cancer; hematological or non-hematological), chronic kidney disease (including renal failure), or chronic liver disease (including hepatic failure). Statistical significance was reached for all features with a displayed p-value.. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Hyperinflammatory Response and Severe COVID-19 Infection
A hyperinflammatory response is characterized by elevated secretion of downstream inflammatory chemokines (MCP-1, IL-8, IP-10), cytokines (IL-6, IL-1), and activation and trafficking of myeloid cells. Hyperinflammatory response is also determined by assessing the levels of the markers of systemic inflammation such as D-dimer and ferritin. Previous studies have revealed that granulocyte-macrophage-colony-stimulating factor (GM-CSF) is one of the early upstream mediators associated with hyperinflammatory immune response and, therefore, an increase in the concentration of circulating GM-CSF indicates severe COVID-19 infection. The levels of C-reactive protein (CRP), which is driven by increased IL-6 following SARS-CoV-2 infection, have also been directly correlated with disease severity.
Scientists have indicated that baseline CRP levels predict the oxygen supplementation requirements in patients hospitalized due to COVID-19 infection as well as worsening organ failure. In the LIVE-AIR study, researchers found that participants that progressed to invasive mechanical ventilation (IMV) or death had mean CRP values consistently above 100 mg/L during their hospital course. Studies have also shown that patients with CRP above 150 mg/L suffer aggressive COVID-19-associated hyperinflammation and are at immediate risk of respiratory failure or death. Recently, researchers have developed models using CRP for COVID-19 treatment guidance. CRP could be used as a biomarker to understand the level of severity of the COVID-19 disease. They hypothesized that earlier treatment of hyperinflammatory immune response with lenzilumab could be made possible by monitoring the patient's CRP levels. Previous studies have also suggested that CRP could be used as a biomarker to determine the extent of hyperinflammatory immune response in COVID-19 patients.
Lenzilumab and COVID-19 Treatment
Lenzilumab is a GM-CSF neutralizing monoclonal antibody assessed to improve the clinical outcomes of hypoxemic hospitalized patients with COVID-19. In the LIVE-AIR phase 3 clinical trial, lenzilumab was administered to COVID-19 patients with decreased oxygen saturation (SpO2≤94%) in room air or in patients who required supplemental oxygen but did not require IMV within two days after hospitalization. Another group of patients was treated with placebo infusion alongside standard treatments that include corticosteroids and remdesivir.
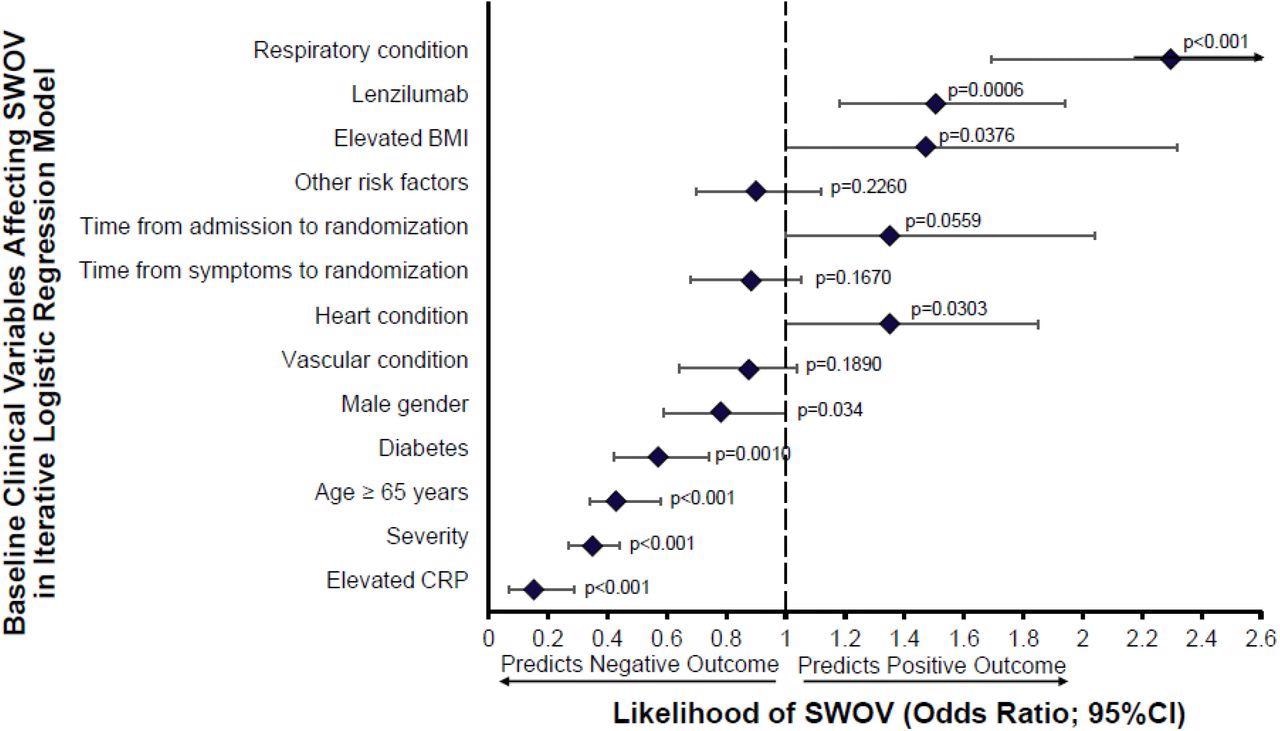
Impact of Baseline Demographics and Risk Factors on Survival with Ventilation Using an Iterative Multivariate Logistic Regression Model. Twelve covariates were included in the model encompassing known risk factors for progression to IMV and/or death by Day 28: Baseline CRP (CRP), disease severity at randomization (severity), respiratory condition (asthma, COPD, interstitial lung disease), age >=65, diabetes (Type 1 or Type 2), lenzilumab (treated or placebo), BMI, time from admission to randomization, time from symptoms to randomization, heart condition (hypertension, coronary artery disease, congestive heart failure), male gender, vascular condition (cerebrovascular disorders, thrombosis or embolism), other risk factors (prior diagnosis of cancer; hematological or non-hematological), chronic kidney disease (including renal failure), or chronic liver disease (including hepatic failure). Statistical significance was reached for all features with a displayed p-value.
In this phase 3 double-blind, placebo-controlled trial, 520 participants were randomized and stratified according to age and disease severity to receive lenzilumab or placebo on Day 0. The study cohort was monitored for twenty-eight days. Scientists reported that lenzilumab treatment significantly enhanced the possibility of survival without invasive mechanical ventilation (SWOV) compared with the controlled group who were treated with a placebo. Marked improvement was reported in patients with baseline CRP<150 mg/L. Scientists observed the lenzilumab decreased the levels of CRP in patients more rapidly than placebo. The levels of CRP were more predictive of SWOV. This study also showed that lenzilumab was well-tolerated without causing any adverse effect in the study cohort.
A recent study has shown that tocilizumab (IL-6 receptor blocker) treatment has significantly improved the outcomes of severely infected COVID-19 patients with a median baseline CRP of 143 mg/L. Although tocilizumab treatment decreased ICU admission or mortality among patients with baseline CRP>150 mg/L, it was not as effective for patients with baseline CRP≤150 mg/L. Additionally, COVID-19 patients with CRP≥200mg/L have been treated with systemic glucocorticoids within 48 hours of hospital admission, but if the CRP were to be less than or equal to 99 mg/L, the use of systemic corticosteroid could be harmful. Therefore, scientists suggested that blocking GM-CSF signaling after monitoring the CRP level could be a beneficial therapeutic approach to prevent progression to severe disease.
Conclusion
Scientists have determined that CRP levels can guide early hyperinflammatory response treatment using lenzilumab, significantly reducing IMV and death due to COVID-19 infection. They indicated increased GM-CSF might occur when a patient progresses towards severe disease. Scientists reported that GM-CSF neutralization using lenzilumab has significantly improved SWOV in the study cohort. In the future, the findings of this study will be further validated by the NIH-sponsored ACTIV-5/BET-B trial.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Temesgen, Z. et al. (2022) Early Lenzilumab Treatment of COVID-19 Patients Using C-Reactive Protein as a Biomarker Improves Efficacy: Results From the Phase 3 ‘LIVE-AIR’ Trial. medRxiv 2021.12.30.21267140; https://doi.org/10.1136/thoraxjnl-2022-218744. https://www.medrxiv.org/content/10.1101/2021.12.30.21267140v1
- Peer reviewed and published scientific report.
Temesgen, Zelalem, Colleen F Kelley, Frank Cerasoli, Adrian Kilcoyne, Dale Chappell, Cameron Durrant, Omar Ahmed, et al. 2022. “C Reactive Protein Utilisation, a Biomarker for Early COVID-19 Treatment, Improves Lenzilumab Efficacy: Results from the Randomised Phase 3 ‘LIVE-AIR’ Trial.” Thorax, July, thoraxjnl-2022-218744. https://thorax.bmj.com/content/early/2022/07/05/thoraxjnl-2022-218744.