The SARS-CoV-2 Omicron variant of concern (VOC) has over 30 mutations in its spike (S) glycoprotein region, which is a crucial antigenic region of the virus against which host cells initiate a neutralizing humoral response. These mutations could potentially reduce the efficacy of current clinically used vaccines and therapeutic antibodies against the Omicron variant.
Study: In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate. Image Credit: Lightspring / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present study, the researchers analyzed the neutralizing power of eight monoclonal antibodies against the Omicron variant and compared these results to the ancestral B.1 BavPat1 D614G strain. The panel of therapeutic monoclonal antibodies included Casirivimab/REGN10933, Imdevimab/REGN10987, Bamlanivimab/LYCoV555, Etesevimab/LY-CoV016, Sotrovimab/Vir-7831, Regdanvimab/CT-P59, Tixagevimab/AZD8895, Cilgavimab/AZD1061, and Evusheld/AZD7742.
The D614G BavPat1 European strain of the B.1 lineage of SARS-CoV-2 was used as a reference to calculate the fold change between the 50% maximal effective concentration (EC50) determined for each virus. EC50 is the antiviral concentration required to inhibit viral ribonucleic acid (RNA) replication by 50%.
The researchers used a standardized RNA yield reduction assay in VeroE6 transmembrane protease serine 2 (TMPRSS2) cells and harvested the cell culture supernatants at 48 hours post-infection (hpi) during the logarithmic growth phase of viral replication. The MAbs were tested in triplicates using two-fold step-dilutions for Cilgavimab and Tixagevimab alone, and in combination from 1,000 to 0.97 ng/mL and from 5,000 to 2.4 ng/mL, respectively. The EC50 was determined by quantifying the amount of viral RNA in the supernatant medium using a quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay.
Study findings
The study results demonstrated that six of these antibodies showed reduced ability to neutralize the Omicron variant. Of the antibodies with neutralizing activity, Sotrovimab/Vir-7831 showed the smallest reduction in neutralizing activity, with a factor change of 3.1. In accordance with preliminary reports, the EC50 of this antibody shifted from 89 to 276 ng/ml as compared to the ancestral B.1 strain.
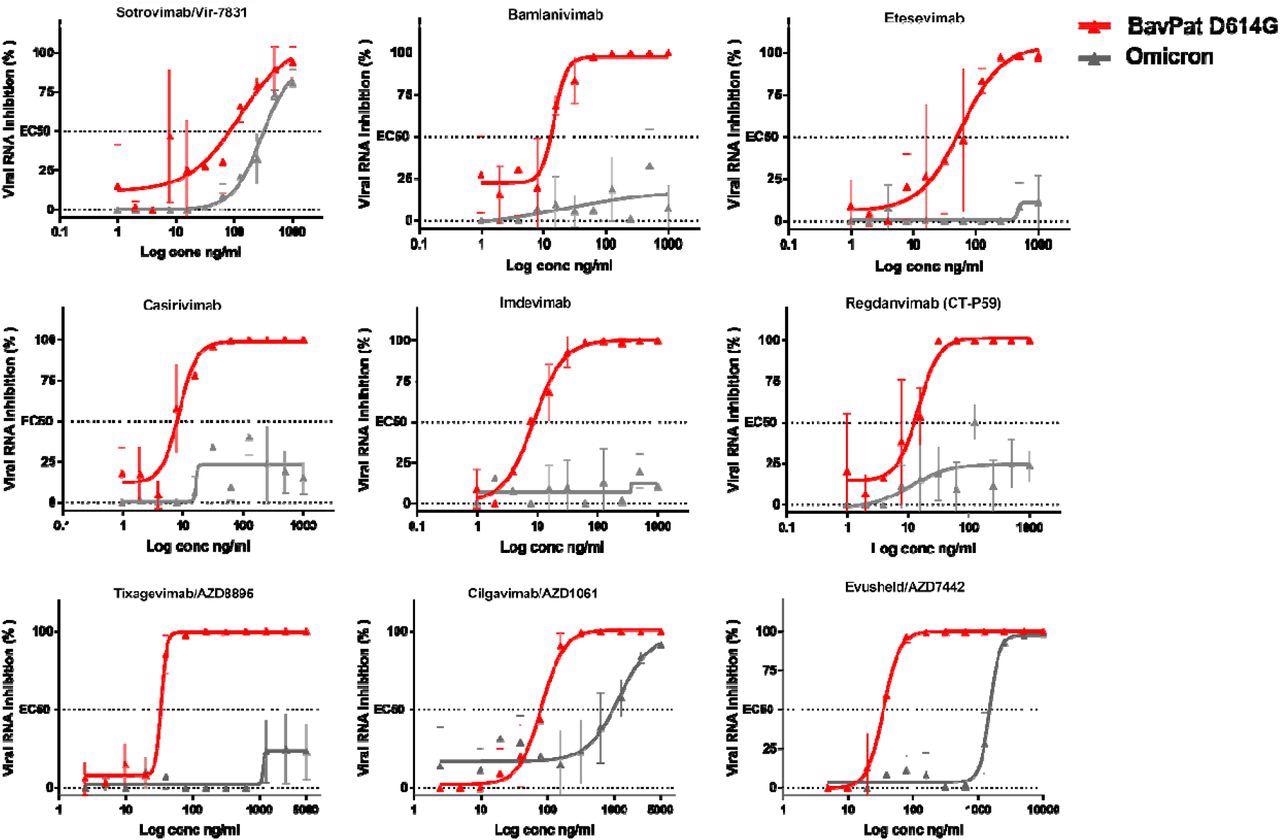
Dose-response curves reporting the susceptibility of the SARS-CoV-2 BavPat1 D614G ancestral strain and Omicron variant to a panel of therapeutic monoclonal antibodies. Antibodies tested: Casirivimab/REGN10933, Imdevimab/REGN10987, Bamlanivimab/LY-CoV555, Etesevimab/LY-CoV016, Sotrovimab/Vir-7831, Regdanvimab/CT-P59, Tixagevimab/AZD8895, Cilgavimab/AZD1061 and Evusheld/AZD7742. Data presented are from three technical replicates in VeroE6-TMPRSS2 cells, and error bars show mean±s.d.
Cilgavimab/AZD1061 showed a reduction in the efficacy of 15.8, which resulted in a 42.6-fold reduction in neutralizing activity for the Evusheld cocktail in which the other antibody, Tixagevimab, did not retain significant activity against Omicron.
With an EC50 value exceeding 5,000 ng/L, Tixagevimab completely lost its neutralizing activity against Omicron. Cilgavimab conserved its neutralizing activity against Omicron with an EC50 shifting from 93 to 1,472 ng/mL, showing a fold change reduction of 15.8.
When Cilgavimab was tested in combination with Tixagevimab, as proposed in the Evusheld/AZD7742 therapeutic cocktail, the EC50 shifted from 35 to 1,488 ng/mL resulting in a fold change reduction of 42.6.
The neutralizing activity of Casirivimab and Imdevimab, Bamlanivimab and Etesevimab, as well as Regdanvimab under the study conditions were not detectable, thus preventing the researchers from calculating their EC50.
The observed reductions in neutralizing activities of the MAbs investigated in the present study should be seen in the context of the actual treatments given to COVID-19 patients. While a single intravenous injection of 500 mg Sotrovimab for the early treatment of COVID-19 infections is registered in the European Union, the Evusheld 300 mg cocktail (150 mg Tixagevimab + 150 mg Cilgavimab, intramuscular) is prescribed during prophylaxis in patients at high risk of developing severe COVID-19.
The number of neutralizing units present in each unit of proposed MAb treatment, based on calculated EC50 values, is expressed in millions of neutralization units 50 per treatment (MNU50).
Conclusions
Although 500 mg of Sotrovimab retained a significant level of neutralizing activity against the Omicron variant, its neutralizing activity against Omicron was about 30% of its activity against ancestral SARS-CoV-2 B.1 strain and about 20% of the neutralizing activity of the Evusheld 300 mg cocktail.
The neutralizing activity of 300 mg of Evusheld was significantly reduced against the Omicron variant. As compared to Sotrovimab 500 mg, this antibody showed about 10% neutralizing activity against Omicron and approximately 2.5% of the neutralizing activity of the Evusheld cocktail against a B.1 strain.
To summarize, these study findings assert the need for a rapid evaluation of the clinical and therapeutic efficacy of the initially proposed 500 mg and 300 mg doses of Sotrovimab and Evusheld, respectively, against the SARS-CoV-2 Omicron variant. Existing doses, as well as those that are currently in development for combination therapies, also need to be modified for the early treatment and prevention of infection with the Omicron variant.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Touret, F., Baronti, C., Bouzidi, H. S., & de Lamballerie, X. (2021). In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate. bioRxiv. doi:10.1101/2022.01.01.474639. https://www.biorxiv.org/content/10.1101/2022.01.01.474639v1.
- Peer reviewed and published scientific report.
Touret, Franck, Cécile Baronti, Hawa Sophia Bouzidi, and Xavier de Lamballerie. 2022. “In Vitro Evaluation of Therapeutic Antibodies against a SARS-CoV-2 Omicron B.1.1.529 Isolate.” Scientific Reports 12 (1). https://doi.org/10.1038/s41598-022-08559-5. https://www.nature.com/articles/s41598-022-08559-5.