
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Various studies have suggested that SARS-CoV-2 infection could lead to neurological symptoms in coronavirus disease 2019 (COVID-19) patients; however, little is known about these neurological manifestations. Microglia are macrophage-like immune cells in the brain and central nervous system (CNS) that maintain brain homeostasis and are known to act in response to injury and inflammation rapidly. In response to immunological stimulus, microglial cells adopt an amoeboid morphology and release interleukins (IL) like IL-1β and IL-6 and tumor necrosis factor–α (TNFα).
Microglia exhibit dual phenotypes when activated. Whereas M1 is considered the classically activated state, M2 is the alternately activated state.
The M1 phenotype is involved in neuroinflammation and is neurotoxic; conversely, the M2 phenotype is neuroprotective. Although much is known about microglial activation and response, more research is required to characterize and understand the microglial host-immune response in patients infected with SARS-CoV-2.
About the study
Researchers investigated the factors driving neuroinflammation and other neurological complications in COVID-19 patients in the present study. This work was primarily conducted in response to several reports of microgliosis, accumulation of immune cells, and microglial nodules in the medulla oblongata and cerebellar dentate nuclei in the brains of deceased COVID-19 patients that arise due to massive activation of microglial cells.
The team investigated whether SARS-CoV-2 can infect human microglial cells by inoculating the human embryonic primary microglia (HMC3) with one multiplicity of infection (MOI) of SARS-CoV-2. In addition, other SARS-CoV-2-susceptible cell lines like Caco-2 and Vero E6 cells were also infected.
Study findings
The researchers found that SARS-CoV-2 infected HMC3 and that this infection triggered the death of HMC3 cells and exhibited a cytopathic effect (CPE). The authors also investigated whether SARS-CoV-2 infection elicited an M1 or M2 phenotype of microglia and analyzed the differentially expressed genes (DEGs) associated with microglial polarization.
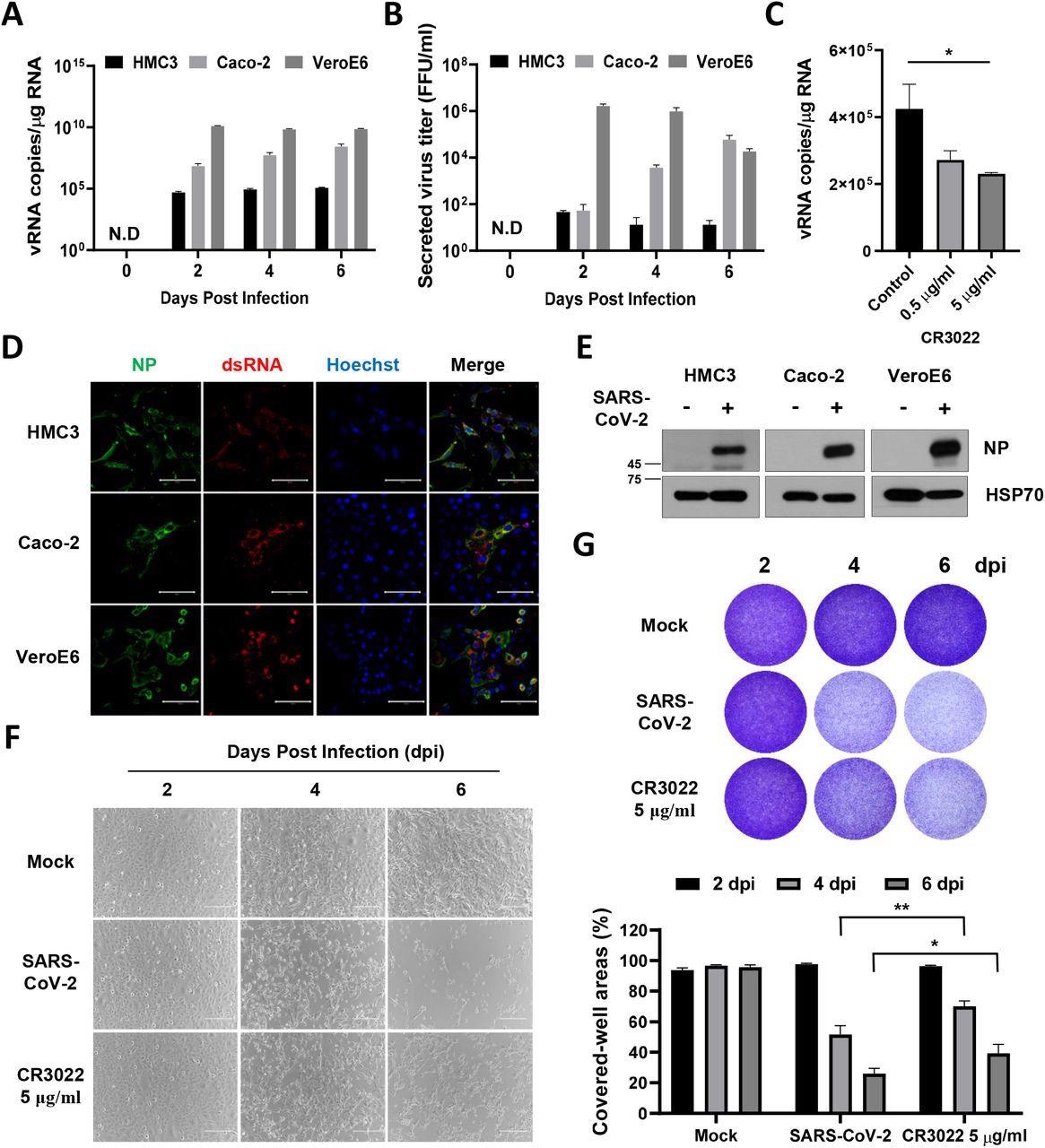
SARS-CoV-2 directly infects human microglia cells, eliciting CPE. A HMC3, Caco-2, and Vero E6 cells were infected with one MOI of SARS-CoV-2. The total cellular RNA was extracted at 2, 4, and 6 dpi to detect the viral RNA of the SARS-CoV-2 NP gene by Quantitative real-time polymerase chain reaction (RT-qPCR). The graph shows viral RNA copies per microgram of total cellular RNA on each day. B The culture media derived from SARS-CoV-2-infected cells were serially diluted and used for focus forming assay. The graph shows the secreted virus titre as focus forming units (FFU). C The graph shows viral RNA copies per microgram of total cellular RNA at 2 dpi after treatment with the increasing amount of CR3022 neutralizing antibody. D Confocal images of SARS-CoV-2-infected HMC3 (top row), Caco-2 (middle row), and Vero E6 (bottom row), demonstrating infection of these cells by immunofluorescence assay with anti-SARS-CoV-2 NP and anti-dsRNA antibodies. Scale bar = 100 μm. E Western blotting of SARS-CoV-2 NP in each infected cell. The 70-KDa heat shock protein (Hsp70) served as the loading control. F Phase-contrast images of the mock or SARS-CoV-2-infected HMC3 in the absence/presence of CR3022 neutralizing antibody at 2, 4, and 6 dpi, indicating cell death as the CPE by microscopy. Scale bar = 200 μm. G Images of crystal violet staining of the mock or SARS-CoV-2-infected HMC3 in the absence/presence of CR3022 neutralizing antibody, plated in the 12-well (upper). The graph shows the percent measurements of crystal violet-stained cell covered areas by ImmunoSpot reader (lower). Statistically significant differences between the groups were determined by Student’s t-test; *P < 0.05; **P < 0.01. Symbols represent mean ± SEM.
An increase in ribonucleic acid (RNA) expression levels of M1 phenotype-related genes like IL-1β, IL-6, and CXCl1 was observed. This suggests that SARS-CoV-2 infection induces a pro-inflammatory activation leading to the M1 phenotype in HMC3.
Further, the researchers determined the mechanism of death of HMC3 cells triggered due to SARS-CoV-2 infection. Western blot analysis revealed that apoptotic proteins associated with both intrinsic and extrinsic pathways of apoptosis were elicited in the SARS-CoV-2-infected HMC3 cells.
Death receptor (DR)-mediated proteins of the extrinsic apoptotic pathway such as Fas, death receptor 4 (DR4), DR5, and TNF receptor 2 (TNFR2) were observed on the Western blots. Moreover, the expression of Bcl-2 (apoptosis suppressor) decreased while that of Bim, Bid, and Bax increased. These findings reveal that SARS-CoV-2 induces cell death of HMC3 cells through both pathways of apoptosis.
Several other RNA viruses such as the Zika virus (ZIKV) and vesicular stomatitis virus (VSV) cause pyroptosis, or inflammatory cell death, in most immune cells. The team ascertained whether pyroptosis is induced by SARS-CoV-2 infection in HMC3 and reported that pyroptosis due to SARS-CoV-2 was not detected in HMC3 cells and concluded that cell death was due to apoptosis.
Transgenic mice (K18-hACE2) expressing human ACE2 with a cytokeratin-18 gene promoter were infected with 2 x 104 plaque-forming units (PFUs) of SARS-CoV-2. After six days of infection, weight loss of about 20% of their body weight was observed in infected mice, and viral RNA was detected in their brains.
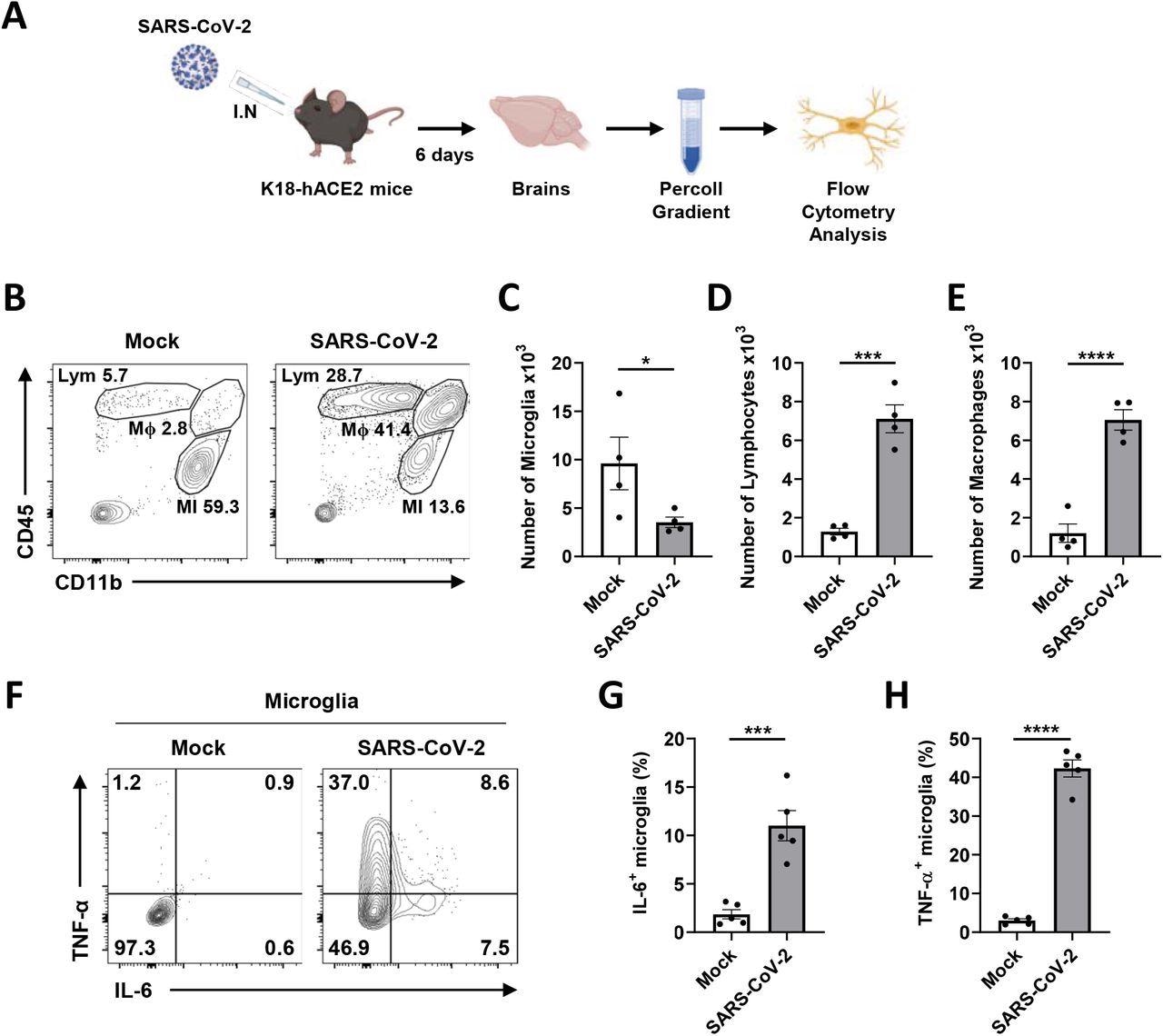
Microglial pro-inflammatory activation and depopulation by SARS-CoV-2 infection in K18-hACE2 mice. A Schematic of the experiment for B to H, created with BioRender.com. After six days, brains of mock or SARS-CoV-2-infected mice were extracted and used for Percoll gradient centrifugation to isolate mononuclear cells containing microglia for the flow cytometry analysis. The cellular surface of isolated mononuclear cells was stained with CD11b and CD45 antibodies. B Representative flow plot gated on leukocytes shows gating for microglia (MI, CD11b+, CD45Low), macrophages (Mϕ, CD11b+, CD45High), and lymphocytes (Lym, CD11b-, CD45High). C-E Bar graphs show the number of microglia (C), lymphocytes (D), and macrophages (E) isolated per brain at 6 dpi. F Representative flow plot gated on microglia shows activated microglia with highly expressed IL-6 and TNF-α to separate activated from ramified microglia. G-H Bar graphs indicate the percentage of activated microglia, highly expressing IL-6 (G) and TNF-α (H). Statistically significant differences between the groups were determined using Student’s t-test; *P < 0.05; ***P < 0.001; ****P < 0.0001. Symbols represent means ± standard error of the mean (SEM).
Conclusions
The present study demonstrates that SARS-CoV-2 infects microglia and induces its subsequent activation and transformation into a pro-inflammatory M1 phenotype. Furthermore, microglial cell death due to SARS-CoV-2 infection is apoptotic, and both extrinsic and intrinsic ways of apoptosis were observed.
An increase in neurotoxic microglia (M1 cells) can lead to other neurological complications, such as the activation of astrocytes and T-lymphocytes, which can cause neuronal damage and death. Furthermore, the blood-brain barrier could be disturbed due to the release of cytokines by microglia that might cause additional neurological symptoms in COVID-19 patients.
In vivo investigations in transgenic mice reported the infection of microglia by SARS-CoV-2, which induced the release of pro-inflammatory cytokines and subsequently caused chronic loss of microglia. These findings suggest a lack of immune response in the brain; therefore, the increased viral replication may lead to different neurological manifestations.
The observations made in this study suggest that microglial cells could be targeted for therapeutic interventions in COVID-19 patients presenting with neurological symptoms.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Jeong, G. U., Lyu, J., Kim, K., et al. (2022). SARS-CoV-2 Infection of Microglia Elicits Pro-inflammatory Activation and Apoptotic Cell Death. bioRxiv. doi:10.1101/2022.01.04.475015. https://www.biorxiv.org/content/10.1101/2022.01.04.475015v1.
- Peer reviewed and published scientific report.
Jeong, Gi Uk, Jaemyun Lyu, Kyun-Do Kim, Young Cheul Chung, Gun Young Yoon, Sumin Lee, Insu Hwang, et al. 2022. “SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death.” Edited by Ujjwal Neogi. Microbiology Spectrum 10 (3). https://doi.org/10.1128/spectrum.01091-22. https://journals.asm.org/doi/10.1128/spectrum.01091-22.