The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen has been circulating since the end of 2019, causing the ongoing coronavirus disease 2019 (COVID-19) pandemic. This led to the development of vaccines to reduce the number of hospitalizations and deaths caused by SARS-CoV-2. The initial reduction in the number of cases with a severe outcome that followed the large-scale rollout of vaccines later flattened out as new variants emerged, often with immune escape capabilities.
Study: Older Adults Mount Less Durable Humoral Responses to a Two-dose COVID-19 mRNA Vaccine Regimen, but Strong Initial Responses to a Third Dose. Image Credit: peterschreiber.media / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Vaccine-induced immunity is weaker among older adults and wanes faster. This has led many countries around the world to target this patient population for a third dose of vaccine in an attempt to boost their immunity levels to protective levels and thus prevent breakthrough infections. These efforts have become increasingly common following the emergence of the SARS-CoV-2 Omicron variant.
The first two vaccines to receive emergency use authorization against the SARS-CoV-2 in the United States were the mRNA vaccines from Pfizer and Moderna, called Comirnaty and Spikevax, respectively.
The current study reports the follow-up of antibody responses in a cohort of adults aged 24 to 98 years, both with and without a prior history of COVID-19. In addition, antibody response levels were measured at six months from when the study participants had received their second vaccine dose, as well as one month after a third dose had been administered.
Study findings
The study results showed that two doses of an mRNA vaccine in older adults produced significantly lower levels of humoral response in terms of both binding and neutralizing antibodies as compared to younger people throughout the period of assessment. After a two-dose mRNA vaccine regimen, the predictors of weak binding antibody response included having more concomitant illnesses.
Conversely, having a longer gap between the first and second doses was linked to a superior binding antibody response, confirming earlier reports. However, binding antibody levels fell faster in older adults, which is likely due to the more significant number of health conditions in this group.
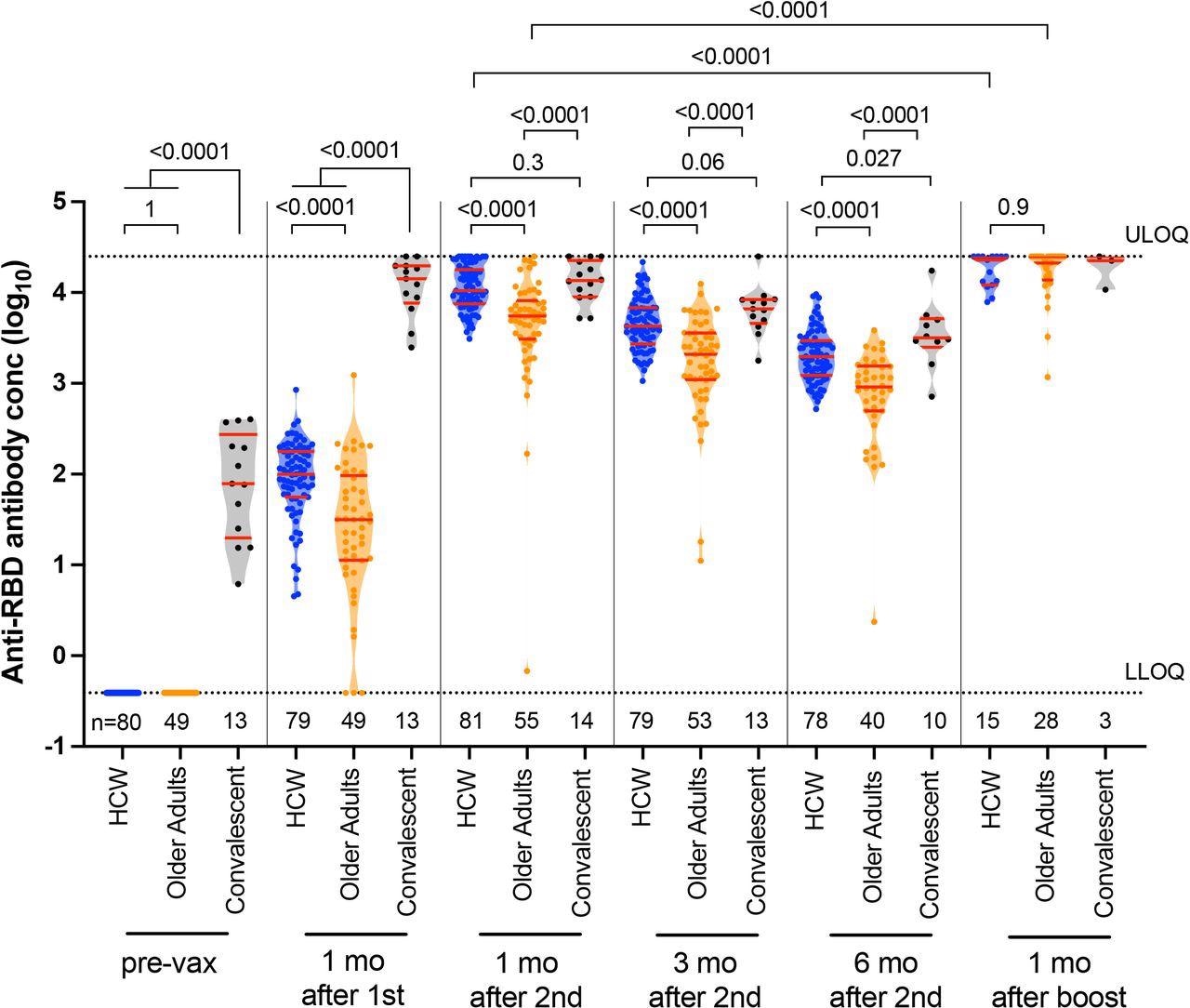
Longitudinal binding antibody responses to spike RBD following one, two, and three COVID-19 vaccine doses. Binding antibody responses to the SARS-CoV-2 spike RBD in serum, in HCW (blue circles) and older adults (orange circles) who were COVID-19 naive at study entry, as well as COVID-19 convalescent individuals (black circles) at six time points: prior to vaccination, one month following the first dose, one, three and six months following the second vaccine dose, and one month following the third or booster vaccine dose. The numbers of participants analyzed are indicated at the bottom of the plot. Red bars and whiskers represent the median and IQR. P-values were computed using the Mann-Whitney U-test (for comparisons between groups) or the Wilcoxon matched-pairs test (for comparisons across time points within a group) and are uncorrected for multiple comparisons. LLOD: lower limit of detection. ULOQ: upper limit of quantification.
Neutralizing antibodies were weak after one dose of the vaccine in previously uninfected individuals, whereas one dose induced neutralizing immunity in those with a history of COVID-19. Two vaccine doses did induce substantial neutralizing immunity in most participants but at lower levels in the elderly adults compared to others.
At six months from receiving the second vaccine dose, neutralizing antibodies had fallen to undetectable levels in most recipients. However, the third dose of vaccine, usually taken six-eight months from the second dose, led to a significant rise in binding and neutralizing antibodies in all groups beyond the peak level achieved with two doses, even in older adults.
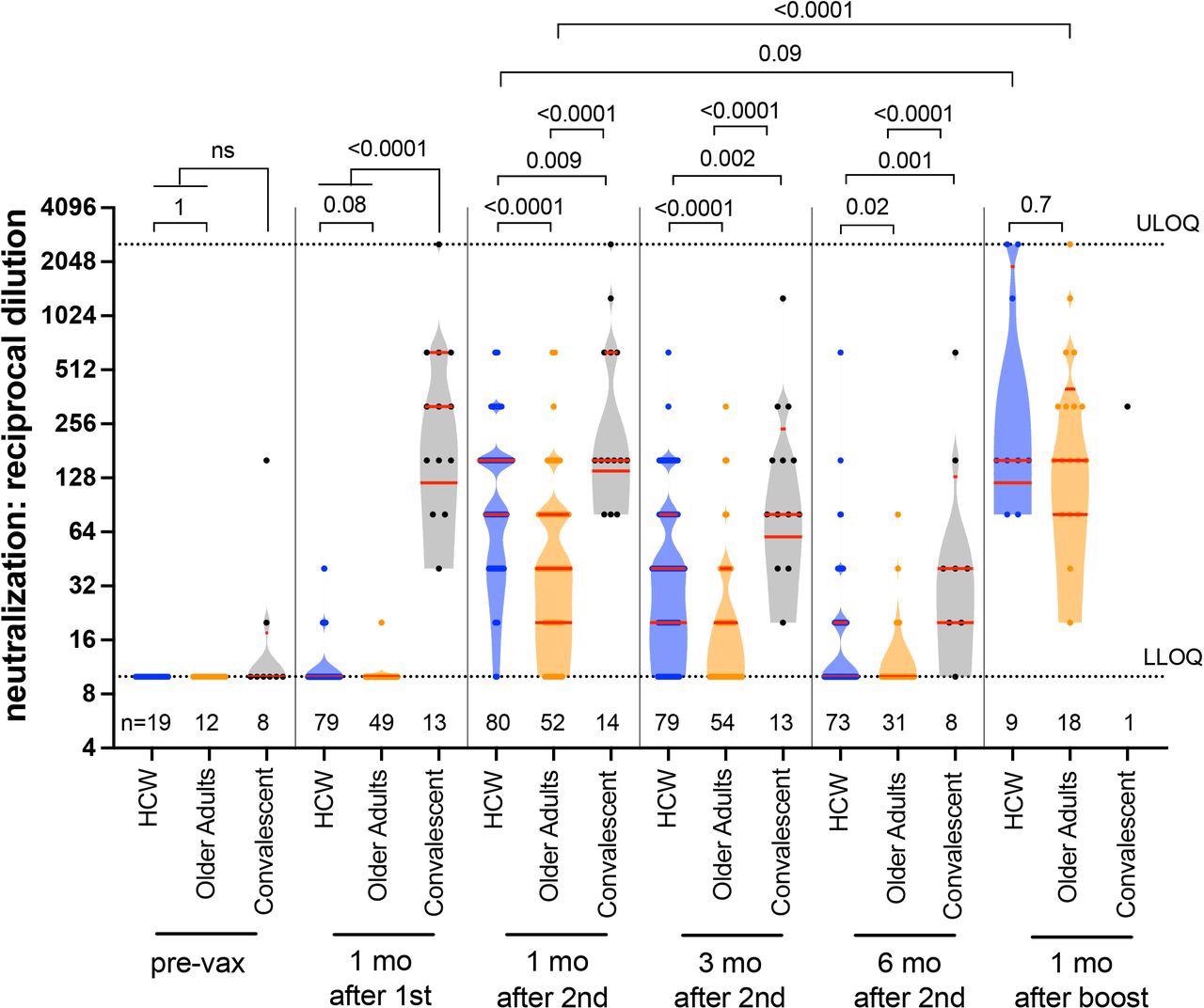
Longitudinal live virus neutralization activity in plasma following one, two and three COVID-19 vaccine doses. Viral neutralization activities, defined as the lowest reciprocal plasma dilution at which neutralization was observed in all triplicate assay wells, in HCW (blue circles) and older adults (orange circles) who were COVID-19 naive at study entry, as well as COVID-19 convalescent participants (black circles) at six time points: prior to vaccination, one month following the first dose, one, three and six months following the second vaccine dose, and one month following the third or booster vaccine dose. Plasma samples showing neutralization in fewer than three wells at a 1/20 dilution were coded as having a reciprocal dilution of 10, corresponding to the lower limit of quantification (LLOQ) in this assay. The highest dilution tested was 1/2560, which corresponds to the upper limit of quantification (ULOQ) in this assay. The numbers of participants analyzed are indicated at the bottom of the plot; note that only a subset of pre-vaccine plasma samples was assayed for this activity. Red bars and whiskers represent the median and IQR. P-values were computed using the Mann-Whitney U-test (for comparisons between groups) or the Wilcoxon matched-pairs test (for comparisons across time points within a group) and are uncorrected for multiple comparisons.
These findings support the recommendation to prioritize older adults for a third or additional booster dose within six months of receiving their second vaccine dose.
Notably, this was not the case with those who were double-vaccinated with an mRNA vaccine after recovering from SARS-CoV-2 infection. In this group, both binding and neutralizing responses were maintained at higher levels than those of naïve vaccinated individuals of all ages.
The waning in the neutralizing antibody response was also slower in this group. This shows that the antibody response elicited by infection followed by vaccination, termed hybrid immunity, is greater and more durable than that induced by vaccination alone.
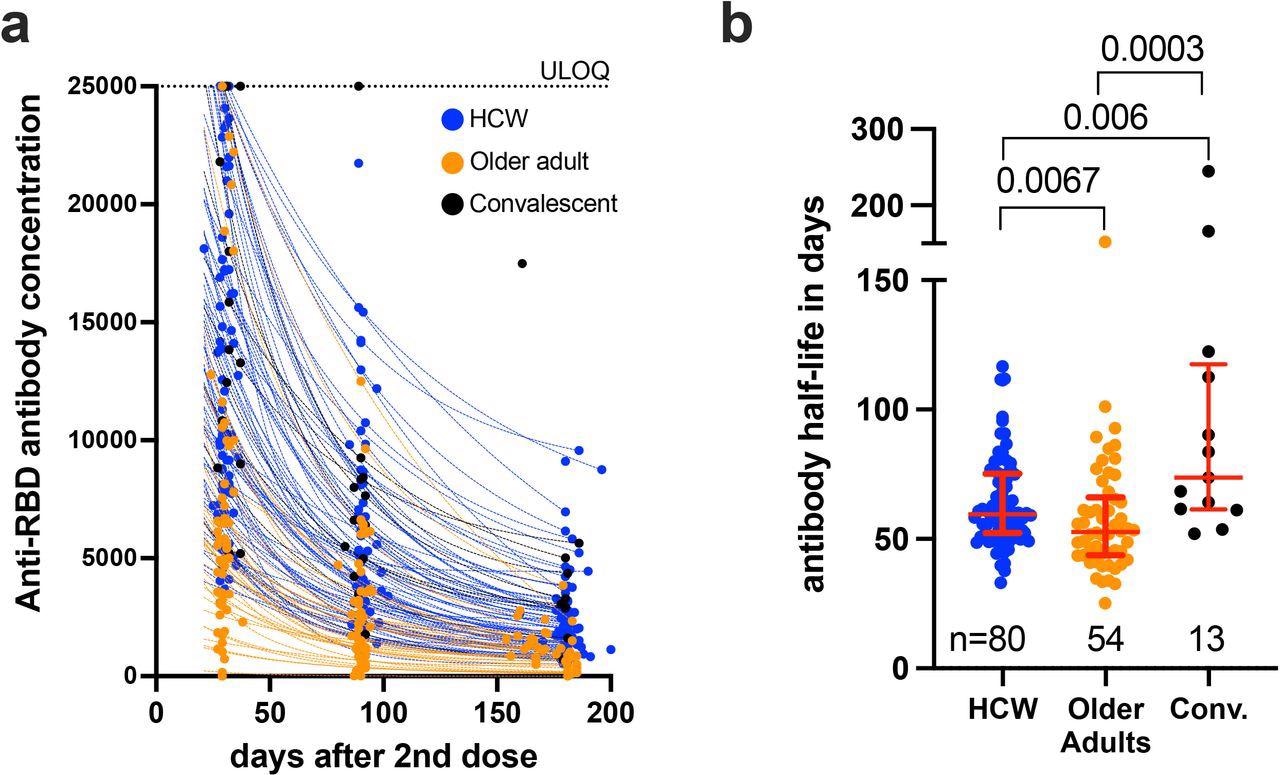
Decay rates and half-lives of serum binding antibody responses to spike RBD following two COVID-19 vaccine doses. Panel A: Temporal declines in serum binding antibody responses to spike-RBD following two COVID-19 vaccine doses in HCW (blue) and older adults (orange) who were COVID-19 naive at study entry, as well as COVID-19 convalescent participants (black circles). ULOQ: upper limit of quantification. Panel B: Binding antibody half-lives following two COVID-19 vaccine doses, calculated by fitting an exponential curve to each participant’s available longitudinal measurements shown in panel A. The numbers of participants analyzed are indicated at the bottom of the plot. Red bars and whiskers represent the median and IQR. P-values were computed using the Mann-Whitney U-test and are uncorrected for multiple comparisons.
The researchers noted that age itself was not a driver of the rapid waning of antibody levels following vaccination, but rather the medical conditions associated with aging,
Implications
The current study supports earlier reports that the third dose of an mRNA vaccine boosts humoral immunity to a greater extent than that achieved with double vaccination among older adults. However, more research is needed to establish that the higher levels of humoral immunity achieved by a third dose are associated with protection from infection and symptomatic disease.
Secondly, the role of T-cell responses to the additional vaccine dose was not included in this study, despite available evidence that this immune response likely has a vital role in providing immunity against emerging SARS-CoV-2 variants.
Longitudinal follow-up and a broader range of testing for neutralizing efficacy against the currently circulating Omicron and other variants will be informative in helping arrive at a conclusion regarding the clinical utility of current COVID-19 vaccines.
“In conclusion, while the observation of strong binding and neutralizing antibody responses to third COVID-19 vaccine doses in older adults, including residents of long-term care, are encouraging, it will be important to closely monitor the decline in these responses over time in this population.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Mwimanzi, F., Lapointe, H., Cheung, P. K., et al. (2022). Older Adults Mount Less Durable Humoral Responses to a Two-dose COVID-19 mRNA Vaccine Regimen, but Strong Initial Responses to a Third Dose. medRxiv. doi:10.1101/2022.01.06.22268745. https://www.medrxiv.org/content/10.1101/2022.01.06.22268745v1.
- Peer reviewed and published scientific report.
Mwimanzi, Francis, Hope R. Lapointe, Peter K. Cheung, Yurou Sang, Fatima Yaseen, Gisele Umviligihozo, Rebecca Kalikawe, et al. 2022. “Older Adults Mount Less Durable Humoral Responses to Two Doses of COVID-19 MRNA Vaccine, but Strong Initial Responses to a Third Dose.” The Journal of Infectious Diseases, May. https://doi.org/10.1093/infdis/jiac199. https://academic.oup.com/jid/article/226/6/983/6583561.