The current coronavirus disease 2019 (COVID-19) pandemic, which is due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused over 5.5 million casualties to date. Despite intensive research into the virus in order to develop vaccines and therapeutic drugs, much remains to be known about the mechanism by how SARS-CoV-2 infection can lead to death.
Study: SARS-CoV-2 Triggers Complement Activation Through Interactions With Heparan Sulfate. Image Credit: CROCOTHERY / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
SARS-CoV-2 is known to replicate rapidly and in high numbers within the infected cell, while cleverly averting host immune system activation through a loophole in the type 1 interferon (IFN) response. At a certain point, the viral particles elicit a hyper-inflammatory response that causes acute respiratory distress syndrome (ARDS), which is characterized by systemic inflammation and multi-organ dysfunction with pulmonary edema.
This process hinges on the activation of the complement system, which is accompanied by high serum levels of C5a and C5b-9, as well as high levels of monocyte and granulocyte CD11b expression. The increased expression of these biomarkers is attributed to C5aR1 activation.
Both the infected airways and injured blood vessels of the host produce complement. Furthermore, complement activation is much higher in those with a predisposition to C5 cleavage, high mannose-binding protein levels, or low CD55 expression.
Multiple pathways of complement activation have been suggested in COVID-19, including both the classical and the alternative pathways of lectins, thereby making this a central mechanism of severe disease following SARS-CoV-2 infection.
One previous study indicated that alternative pathway complement activation was initiated by viral binding to a cell surface glycoprotein called heparan sulfate. This binding led to the release of factor H-dependent complement suppression from inhibition. When cells lacking complement inhibitors were first exposed to the SARS-CoV-2 spike protein in normal human serum, complement deposition occurred and ultimately led to cell toxicity.
When these cells were not present, neither spike-dependent cytotoxicity nor complement activation occurred in the serum. This indicated the need for inhibition of complement. Similar effects were achieved by adding heparan sulfate or factor H.
Other researchers have demonstrated lectin pathway complement activation by the viral spike or nucleocapsid proteins. The current study aimed to provide strong evidence that the SARS-CoV-2 spike protein is capable of directly activating the complement system, thus triggering fatal outcomes of infection.
Study findings
The researchers used live virus in anticoagulated human blood and assessed the extent to which it was able to activate complement, while also measuring the resulting complement function. Specific antagonists of other pathways, such as factor B, C3, and C5 antagonists, were used to inhibit complement activation through these routes. None of the blood samples contained SARS-CoV-2 antibodies, thus eliminating any antibody-mediated classical pathway activation of complement.
C3, C5, and heparan sulfate inhibitors are not capable of passing through the cell membrane. Therefore, it is clear that SARS-CoV-2 interacts with heparan sulfate to activate the alternative pathway leading to the deposition of plasma complement.
The next step was to test for complement functionality following activation. C5aR1 is a complement receptor molecule that enters the host myeloid cell following the stimulation of the cell by activated C5, while CD11b expression at the cell surface is increased. The results show that depending on the infective dose, monocyte activation occurred.
The use of C5/C5aR1 antagonists like eculizumab and PMX205, respectively, led to the suppression of C5aR1 internalization and CD11b upregulation following exposure to the viral spike in the granulocyte species neutrophils and eosinophils to equal extents. In monocytes, the effect was partial and confined to C5aR1 internalization, thereby indicating that C5 activation is not key to these processes in monocytes. This is supported by the fact that C5 activation leads to immune activation within an hour, rather than the 24-hour response seen in the current model.
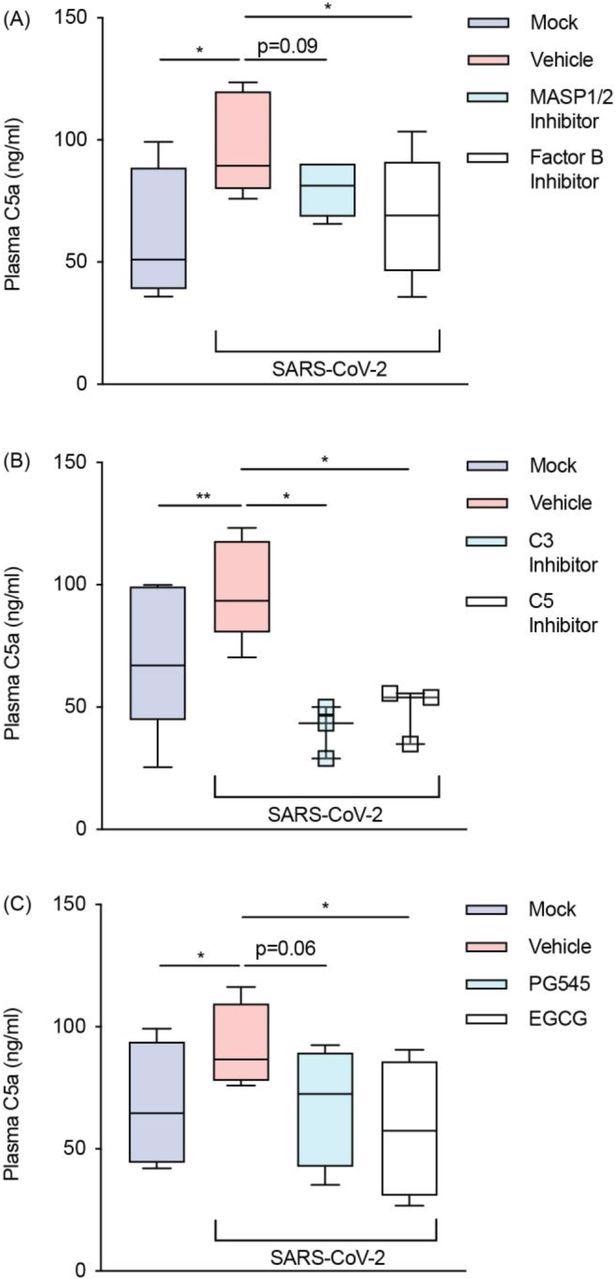 SARS-CoV-2 activates the alternative pathway through interactions with heparan sulfate. Plasma C5a levels were assessed with an ELISA in SARS-CoV-2 inoculated lepirudin-anticoagulated human blood pre-treated with various inhibitors and mimetics. These included antagonists for (A) MASP1/2 (SFMI-1 10μM) and factor B (LNP023 10μM) (n = 5); (B) C3 (Compstatin 20μM) and C5 (Eculizumab 100μg/mL) (n = 3); and (C) heparan sulfate (EGCG 100μM) as well as a mimetic for heparan sulfate (PG545 100μg/mL) (n = 4). SARS-CoV-2 was inoculated at MOI 1.0 for 24 hours. Boxes depict medians and inter-quartile ranges and have Tukey whiskers. Where this was not possible, individual values are presented instead. MOI = multiplicity of infection; * P<0.05, ** P<0.01, using a paired one-tailed t-test. All blood donors were seronegative for anti-SARS-CoV-2 antibodies
SARS-CoV-2 activates the alternative pathway through interactions with heparan sulfate. Plasma C5a levels were assessed with an ELISA in SARS-CoV-2 inoculated lepirudin-anticoagulated human blood pre-treated with various inhibitors and mimetics. These included antagonists for (A) MASP1/2 (SFMI-1 10μM) and factor B (LNP023 10μM) (n = 5); (B) C3 (Compstatin 20μM) and C5 (Eculizumab 100μg/mL) (n = 3); and (C) heparan sulfate (EGCG 100μM) as well as a mimetic for heparan sulfate (PG545 100μg/mL) (n = 4). SARS-CoV-2 was inoculated at MOI 1.0 for 24 hours. Boxes depict medians and inter-quartile ranges and have Tukey whiskers. Where this was not possible, individual values are presented instead. MOI = multiplicity of infection; * P<0.05, ** P<0.01, using a paired one-tailed t-test. All blood donors were seronegative for anti-SARS-CoV-2 antibodies
Implications
The study findings indicate that, as expected, severe COVID-19 is associated with intense activation of the complement system through the alternative pathway, as demonstrated following its activation by the SARS-CoV-2 spike through its binding to the membrane heparan sulfate molecule. However, these ex vivo findings are not a complete reflection of actual infection-associated events.
"Complement activation during COVID-19 is probably a multifactorial phenomenon driven by multiple complement pathways, non-specific DAMP release, genetic susceptibility to complement activation, and local complement synthesis.”
Given the slow progression of disease in severe COVID-19, with leukocytes showing raised CD11b expression levels, the current study points to the involvement of heparan sulfate and the alternative pathway of complement activation in severe disease.
Complement activation occurs in tissues where SARS-CoV-2 replicates, rather than primarily in the plasma, which shows secondary activation. In order to inhibit this activation pharmaceutically, the drug must show the ability to permeate the tissues extensively. This may explain the low rate of success in earlier clinical drug trials focusing on this area.
In fact, scientists describe the virus as “an organ-based activator of complement that poses unique challenges to drug development.” Even so, complement activation does not appear to be a primary action of bound heparan sulfate. It is rather a post-translational modification that leads to specific protein-protein interactions to result in an increase in factor H levels on the host cells, thus suppressing complement activation.
The primary use of heparan sulfate by SARS-CoV-2 is to bind to the angiotensin-converting enzyme 2 (ACE2) host cell receptor and subsequently gain entry to the cell. The secondary effect is the release of the inhibitory factor H on host cells from suppression, causing complement activation. The spike protein binds not only to heparan sulfate but to syndecan 1-4 and other glycoproteins.
The spike can prevent anticoagulant activity as well. Thus, spike-heparan sulfate binding may yield other targets for drug treatment of this infection. The results thus “support the use of targeted anti-complement treatments in severe COVID-19.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Lo, M. W., Amarilla, A. A., Lee, J. D., et al. (2022). SARS-CoV-2 Triggers Complement Activation Through Interactions With Heparan Sulfate. bioRxiv. doi:10.1101/2022.01.11.475820. https://www.biorxiv.org/content/10.1101/2022.01.11.475820v1.
- Peer reviewed and published scientific report.
Lo, Martin W, Alberto A Amarilla, John D Lee, Eduardo A Albornoz, Naphak Modhiran, Richard J Clark, Vito Ferro, et al. 2022. “SARS-CoV\n -2 Triggers Complement Activation through Interactions with Heparan Sulfate.” Clinical & Translational Immunology 11 (8). https://doi.org/10.1002/cti2.1413. https://onlinelibrary.wiley.com/doi/10.1002/cti2.1413.