In a recent study published in the United States Centers for Disease Control and Prevention (CDC) Emerging Infectious Diseases journal, researchers investigate the cross-reactivity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with pre-pandemic malaria-exposed sera samples collected from Cambodia.
Background
Apart from the SARS-CoV-2 screening of healthcare workers in two urban hospital-based settings, no other SARS-CoV-2 serosurveys from the Greater Mekong Subregion (GMS) of Cambodia are available. Serosurveys of SARS-CoV-2 provide valuable insights about high-risk populations or disease management measures against the coronavirus disease 2019 (COVID-19).
However, the results of these serological assays showed variations that may be due to differences in assay methodologies and hypothesized cross-reactivity of previous common cold-type respiratory coronaviruses, uncharacterized beta coronaviruses, or Plasmodium infections in rural GMS populations with SARS-CoV-2.
Since the inhabitants of GMS are exposed to a diverse number of pathogens, the estimation of SARS-CoV-2 seroprevalence in this population is significant.
About the study
In this retrospective study, the researchers analyzed 528 malaria-infected serum samples for IgG reactivity towards the SARS-CoV-2 spike and receptor-binding domain (RBD) proteins using enzyme-linked immunosorbent assay (ELISA). The anonymized serum or plasma samples from the residents of Cambodia, who were infected with malaria between 2005-2011, stored in a biobank after malaria research studies were used for the study.
Since six other coronaviruses including 229E, HKU1, OC43, NL63, SARS-CoV-1, and the Middle East respiratory syndrome coronavirus (MERS-CoV), are able to infect humans, highly-specific ELISA was used to analyze SARS-CoV-2 in the current study.
A positive ELISA test with 100% sensitivity and specificity was expected once the seroreactivity towards the SARS-CoV-2 spike and RBD proteins were above the cutoff values.
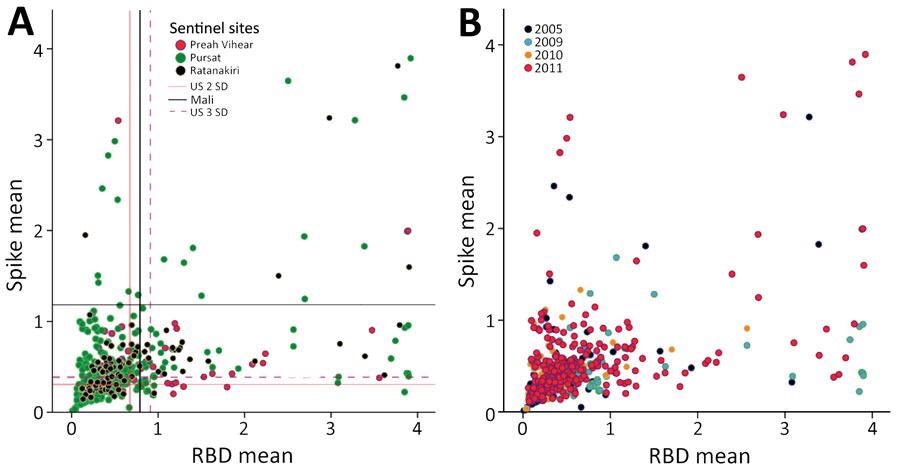
Mean antibody intensity in arbitrary ELISA units to spike and RBD in serum samples from pre-pandemic, malaria-positive rural persons in Cambodia, 2005–2011. A) Provinces indicated by color: Preah Vihear (pink), Pursat (green), Ratanakiri (black). B) Years indicated by color: 2005 (purple), 2009 (turquoise), 2010 (orange), and 2011 (pink). RBD, receptor-binding domain.
A subset of the study population was analyzed to confirm the higher-than-expected seropositivity of samples towards SARS-CoV-2 using a commercially validated SARS-CoV-2 Spike S1-RBD IgG ELISA Detection Kit. The seroreactivity of a subset of samples towards coronaviruses OC43 and HKU1 was also determined.
The serum antibodies’ relationship between Plasmodium falciparum (P. falciparum) apical membrane antigen 1 (AMA-1) and P. falciparum Pfs25 protein (Pfs25) with SARS-CoV-2 spike, RBD, and nucleocapsid proteins were assessed in 289 samples.
The neutralizing capacities of the detected SARS-CoV-2 antibodies were determined using neutralization and surrogate virus neutralization assays.
Study findings
The results of the ELISA experiments showed that as compared to the six other coronaviruses, SARS-CoV-2 had varying levels of spike protein sequence homology. The highest and lowest levels were observed in SARS-CoV-1 and the common cold coronavirus HKU1, respectively.
The pre-pandemic malaria-positive samples had seropositivity ranging from 4.4% to 13.8% to both SARS-CoV-2 spike and RBD antigens.
Out of the 24 SARS-CoV-2-seronegative and 11 seropositive individuals by the in-house ELISA, 18 and nine tested seronegative and seropositive, respectively, using the commercial ELISA kit. The in-house and commercial ELISAs had an overall concordance of 77.1% and a higher-than-expected positivity rate.
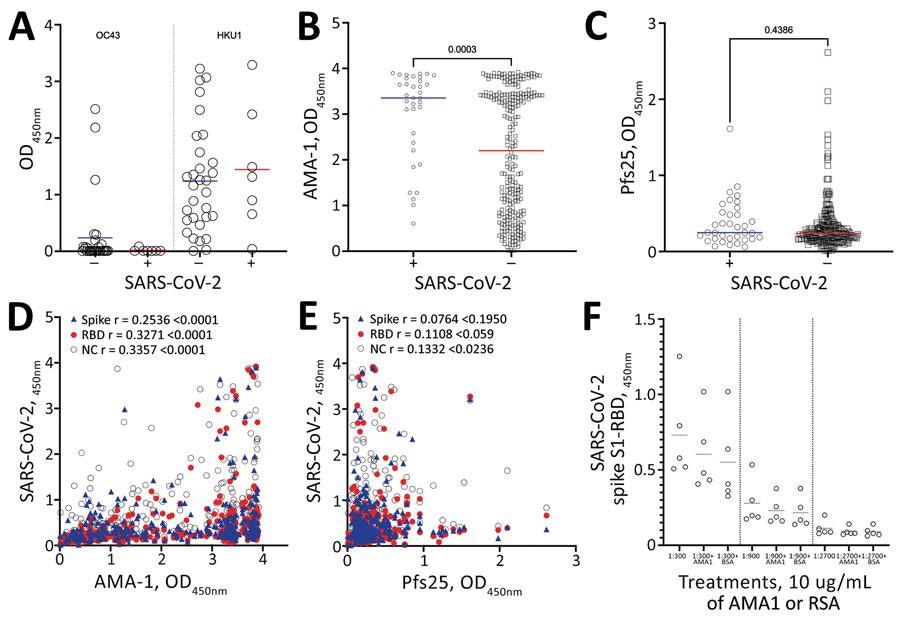
Mean antibody levels in prepandemic serum samples from malaria-positive rural persons in Cambodia, 2005–2011, to A) common cold OC43 and HKU1 viruses, B) Plasmodium falciparum AMA-1 and C) P. falciparum Pfs25 protein by SARS-CoV-2 serosurvey statuses. D–E) Correlation of mean IgG levels of AMA-1 and Pfs25 against Spike (blue triangles), RBD (red circles) and NC (open circles) IgG levels in pre pandemic serum samples from malaria-positive rural persons in Cambodia. F) OD levels of RBD protein after preincubation of serum samples with 10mg/mL of AMA-1 or BSA. AMA-1, apical membrane antigen 1; BSA, bovine serum albumin; NC, nucleocapsid; OD, optical density; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The cohort subgroups had comparable IgG levels against coronaviruses HKU1 and OC43, regardless of the SARS-CoV-2 serostatus.
Interestingly, SARS-CoV-2 seropositive individuals had a higher level of AMA-1 antibodies than the seronegative subjects. However, Pfs25 antibodies did not vary in association with the SARS-CoV-2 seropositivity. Although feeble, a statistically significant positive correlation was observed between the SARS-CoV-2 spike and RBD with AMA-1 IgG.
The IgG levels of AMA-1 and the SARS-CoV-2 nucleocapsid protein positively correlated in the samples. In contrast, the IgG levels of Pfs25 and SARS-CoV-2 nucleocapsid protein had a weak correlation.
Preincubated serum samples with 10 mg/mL of AMA-1 or bovine serum albumin (BSA) did not show any significant change in reactivity towards SARS-CoV-2 spike S1-RBD.
The neutralization assay of the 21 samples with high reactivity to SARS-CoV-2 total IgG indicated no neutralizing activity, despite high levels of antibodies against the spike and RBD proteins. An identical result was seen with the surrogate virus neutralization test targeting RBD-angiotensin converting enzyme 2 (ACE2) interaction.
Conclusions
The current study summarized that approximately 4-14% of pre-pandemic serum samples from malaria-infected individuals had non-neutralizing antibodies against the SARS-CoV-2 spike and RBD antigens. The current findings were in line with a recent study from Africa addressing the correlation between antimalarial humoral immunity and higher SARS-CoV-2 seroreactivity.
The higher SARS-CoV-2 cross-reactivity might be associated with exposure to uncharacterized beta coronaviruses or regular spillover events and cannot be neglected in the case of Cambodia, since 50-80% of GMS residents are from rural areas. Therefore, careful calibrations of serologic testing are required in the national and subnational SARS-CoV-2 serosurveys.
Moreover, economically feasible competitive ELISAs such as surrogate virus neutralization assays are recommended in large-scale serosurveys as compared to costly neutralization assays with live viruses.
Journal reference:
- Manning, J., Zaidi, I., Lon, C., et al. (2022). SARS-CoV-2 Cross-Reactivity in Prepandemic Serum from Rural Malaria-Infected Persons, Cambodia. CDC Emerging Diseases. doi:10.3201/eid2802.211725.