
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The signals generated during the primary immune response, as perceived by both B- and T- lymphocytes, leading to the induction of immune responses under the adaptive arm of the immune system. The immune system has also evolved to elicit a specific functional response to fight infections and re-infections. Immune memory is influenced by various factors such as the site of antigenic encounters and the number of exposures to the antigen, as well as specific inflammatory signals.
Hybrid/super immunity is a concept that is defined as the immune response generated by prior SARS-CoV-2 infection and subsequent coronavirus disease 2019 (COVID-19) vaccination. It has been proposed that this hybrid form of immunity can offer enhanced protection against both re-infections and severe forms of COVID-19, which neither infection nor vaccination can individually provide.
Some studies have indeed reported an improved neutralizing potency of circulating antibodies. However, the specific changes in the cellular immune responses associated with this hybrid state of immunity are unknown. It also remains unclear whether a subsequent activation of immune memory in vaccinated-only individuals could produce similar effects.
About the study
In the present study, researchers investigate how vaccine-induced immune memory is influenced by previous SARS-CoV-2 infections. SARS-CoV-2-specific antibodies and memory lymphocytes in circulation were tracked in naive (N) and previously infected (PI) subjects following three doses of COVID-19 vaccination with either BNT162b2 or mRNA-1273 vaccines.
Specifically, the receptor-binding domain (RBD)-directed antibodies and B-cells, as well as spike protein (S)-specific CD4+ T-cells were analyzed in infected patients for over two years. The targets of SARS-CoV-2-directed vaccines were also examined to ascertain the influence of vaccination on immunity.
Study findings
The researchers observed that PI individuals had B-lymphocytes, as well as both immunoglobulin G (IgG) and IgA antibodies specific to SARS-CoV-2 RBD before vaccination. Two doses of COVID-19 vaccines led to a robust humoral response in both N and PI individuals; however, the PI subjects had elevated numbers of RBD-specific antibody titers and plasmablasts than N individuals after three and six months of vaccination.
After the third vaccine dose, both N and PI individuals elicited an equal immune response. However, the antibody response in N individuals after three SARS-CoV-2 antigen exposures did not match that in PI individuals after their third exposure, which corresponds to their second dose of vaccine.
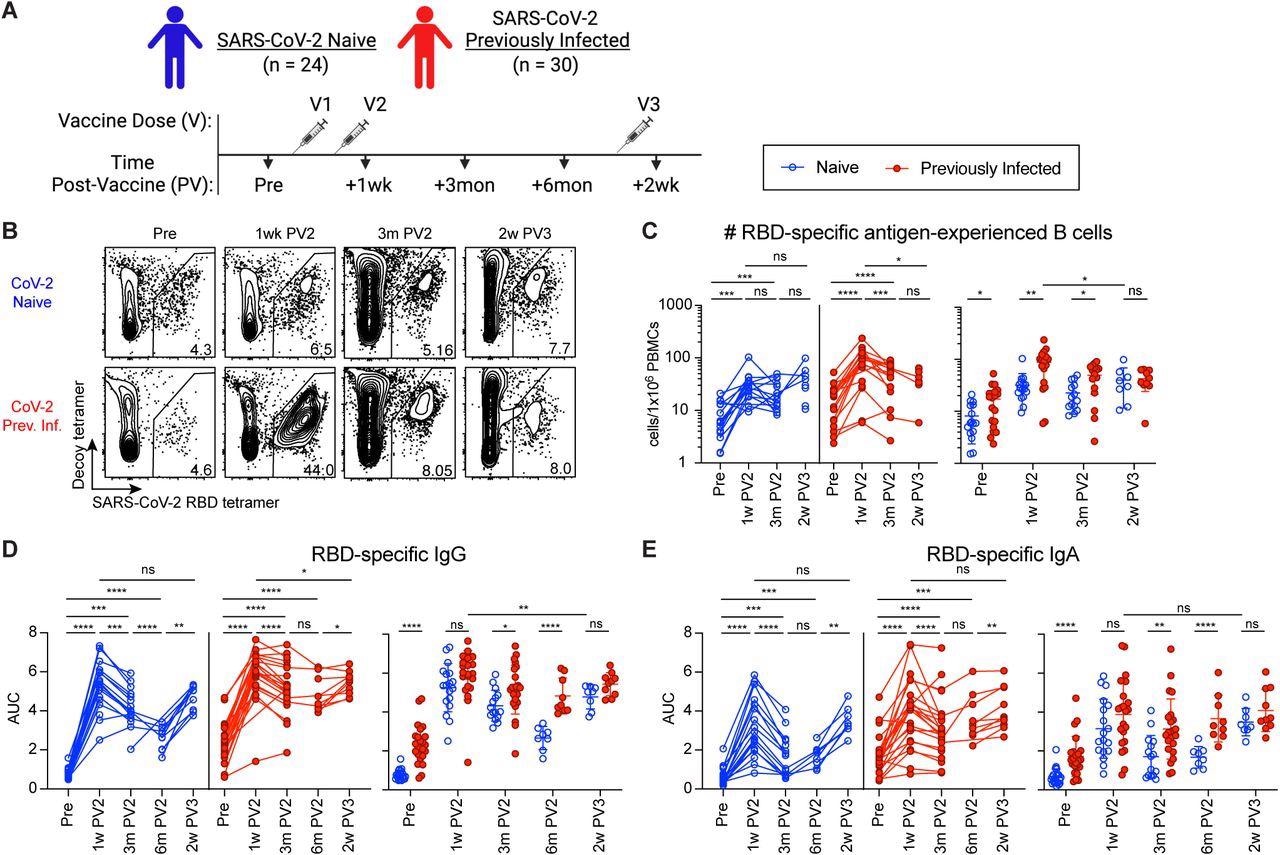
The greater humoral response to vaccination in SARS-CoV-2 previously infected compared to naive individuals is recovered by third vaccination. (A) Timeline of blood draws from SARS-CoV-2 naive (N) and previously infected (Prev. Inf., PI) analyzed in this study relative to vaccinations. (B) Representative gating on CD19+CD38lo B cells for RBD-tetramer+Decoy- SARS-CoV-2 RBD-specific B cells from N and PI (Prev. Inf.) PBMCs at the indicated time points pre-vaccination (Pre), 1 week post-2nd vaccination (1w PV2), 3 months post-2nd vaccination (3m PV2) and 6 months post-2nd vaccination (6m PV2). (C) Number of RBD-specific antigen-experienced (ag-exp.) B cells (CD21+CD27+ and CD21-CD27+/-) in N (blue) and PI (red) PBMCs at the indicated time points. (D and E) ELISA area under the curve (AUC) for RBD-specific IgG (D) and IgA (E) plasma antibody from N and PI individuals at indicated time points. Statistics for unpaired data determined by 2-tailed Mann-Whitney tests and, for paired data, by 2-tailed Wilcoxon signed-rank tests: not significant (ns), *P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001. Error bars represent mean and SD.
PI individuals demonstrated the presence of CD4+ T-cell activity against membrane (M), nucleocapsid (N), and S proteins before vaccination. Double vaccination generated an equivalent number of S-specific CD4+ T-cells in all participants, irrespective of previous SARS-CoV-2 exposure, which lasted for at least 18 months.
There was no difference in the frequency of S-reactive central memory T-lymphocytes (CM) in either cohort before vaccination. However, PI subjects retained effector memory (EM) and follicular helper T (Tfh) cells.
After two vaccinations in both groups, an increase in effector and memory T-cells was noted and the pre-vaccination numerical difference between the two cohorts was normalized. However, a third dose did not translate into an increased population of effector and memory T-cells in the subjects of either group.
The authors found that PI individuals were more capable of neutralizing SARS-CoV-2 variants of concern (VOCs) like the Beta and Omicron variants after two doses of vaccine than N individuals. It was proposed that a third dose is needed for N individuals to attain the VOC neutralizing potency of PI individuals.
Robust induction of interferon-γ (IFN-γ)-secreting S-reactive CD4+ T-cells and interleukin-10 (IL-10)-producing CD4+ T-cells was observed after vaccination in both groups; however, these levels were higher in the PI group than the N group after both second and third vaccinations. Functionally distinct subsets of CD4+ T-cells emerged after vaccination in both PI and N cohorts, which differed based on prior SARS-CoV-2 infection.
Conclusions
The observations made in this study delineated the humoral and cellular responses induced in PI and N individuals. The differences in humoral responses between both cohorts were normalized after the booster vaccine shot.
The cytokine profile of CD4+ T-cells in PI individuals exhibited a unique profile of IFN-γ- and IL-10-secreting cells that were unattainable by multiple vaccinations in N individuals, thereby suggesting that hybrid immunity is imprinted on responding cells during the initial SARS-CoV-2 infection. A third dose of the COVID-19 vaccine did not increase memory T-lymphocytes, thus indicating the saturation of immune memory against SARS-CoV-2. These findings can be used to optimize the current vaccination strategy and design specific vaccines for SARS-CoV-2 variants in the future.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Rodda, L. B., Morawski, P. A., Pruner, K. B., et al. (2022). Imprinted SARS-CoV-2-Specific Memory Lymphocytes Define Hybrid Immunity. medRxiv. doi:10.1101/2022.01.12.22269192. https://www.medrxiv.org/content/10.1101/2022.01.12.22269192v1
- Peer reviewed and published scientific report.
Rodda, Lauren B., Peter A. Morawski, Kurt B. Pruner, Mitchell L. Fahning, Christian A. Howard, Nicholas Franko, Jennifer Logue, et al. 2022. “Imprinted SARS-CoV-2-Specific Memory Lymphocytes Define Hybrid Immunity.” Cell 185 (9): 1588-1601.e14. https://doi.org/10.1016/j.cell.2022.03.018. https://www.cell.com/cell/fulltext/S0092-8674(22)00328-2.