The rapid transmissibility of the SARS-CoV-2 Omicron VOC has caused a surge in coronavirus disease (COVID-19) cases and related hospitalizations globally. The efficiency of the mRNA-1273 vaccine in neutralizing the Omicron VOC has been analyzed by numerous studies. However, the difference in mRNA-1273 dosages required to effectively neutralize Omicron in different age groups requires more investigation.
Study: mRNA-1273 Vaccine-elicited Neutralization of SARS-CoV-2 Omicron in Adolescents and Children. Image Credit: rafapress / Shutterstock.com

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
In the present study, the researchers assess the neutralization efficacy of the mRNA-1273 vaccine against Omicron infections in children, adolescents, and adults. Serum samples analyzed for this study were collected from three ongoing clinical trials including samples from adults aged 18 years and above from Phase III of the Coronavirus Efficacy (COVE) trial, adolescents aged 12 to 17 years from Phase II and III of the TeenCOVE study, and children between the ages of six and 12 years from Phase II and III of the KidCOVE study.
All adults and adolescents in the current study received two doses of 100 micrograms. (µg) of the mRNA-1273 vaccine as a part of the primary vaccination series, while the children received two doses of 50 µg mRNA-1273.
Sample selection for the study was conducted so that each vaccine group had 20 participants who had tested negative for SARS-CoV-2 at baseline by reverse transcription-polymerase chain reaction (RT-PCR) assay and Roche Elecsys serology test. Omicron neutralization titers in the serum samples collected on day one before the first dose and in the samples collected a month after administration of the second vaccine dose were analyzed.
These titers were compared to the corresponding titers analyzed against the prototypic SARS-CoV-2 wild-type (D614G) strain. A neutralizing assay based on lentivirus pseudovirus was performed to measure neutralizing titers at day one and a month after administration of the second vaccine dose.
Study findings
The median age of study participants was 57.8 years for adults, 13.9 years for adolescents, and 8.8 years for children. About 95% of the adults had significant Omicron neutralizing antibody titers four weeks after the second dose of the mRNA-1273 vaccine. A reduction of 28.8-fold in the geometric mean ID50 titer (GMT) was observed in the Omicron neutralizing titers as compared to the D614G strain.
Omicron neutralization was observed in 100% of the adolescents in the vaccine group when measured four weeks after the second dose of mRNA-1273, while the GMT reduced by 11.8-fold as compared to D614G GMT. In children, the Omicron neutralization was observed in 100% of individuals four weeks after the second mRNA-1273 dose; however, the Omicron GMT was 22.1-fold lower than the D614G-GMT.
The GMTs of D614G and Omicron in adolescents were 1.5-fold and 3.8-fold higher than that of adults, respectively. Comparatively, the GMTs of D614G and Omicron in children were found to be 2.0-fold and 2.5-fold higher as compared to adults, respectively.
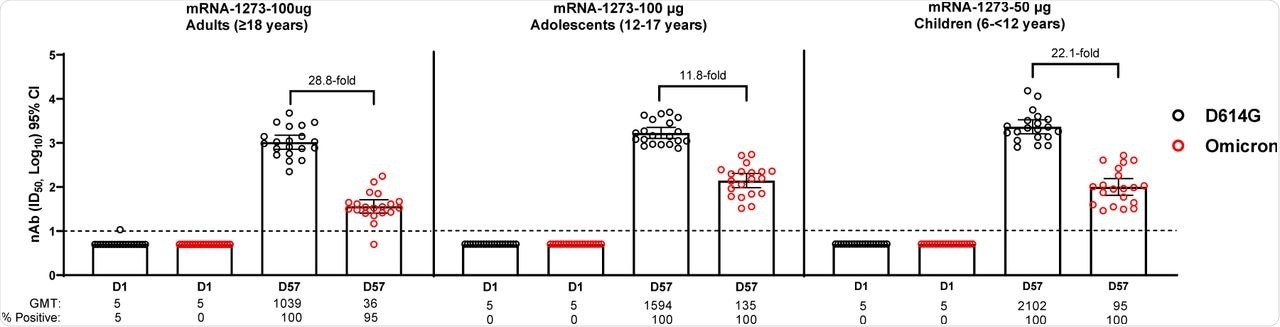
Neutralization of D614G and Omicron SARS-CoV-2 Pseudoviruses by Sera from mRNA-1273 Primary Vaccination Recipients.
Conclusions
Based on the study findings, neutralizing titers against D614G and Omicron four weeks after the second dose of mRNA-1273 vaccines were significantly higher in children and adolescents than in adults. Furthermore, Omicron neutralization titers in adults were less when compared to D614G neutralization titers in children and adolescents.
The researchers believe that the efficacy of the mRNA-1273 vaccines against the SARS-CoV-2 Omicron VOC in children and adolescents requires more thorough research in the future. The efficiency of the vaccine in diverse populations over longer periods should also be analyzed in ongoing clinical trials.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.