
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The emergence of SARS-CoV-2 variants of concern (VOC) throughout the coronavirus disease 2019 (COVID-19) pandemic has led to multiple waves of infection.
The Omicron (B.1.1.529) VOC has the highest number of mutations in the spike protein as compared to any other VOC. These mutations are largely responsible for their high transmissibility and decreased susceptibility to therapeutic monoclonal antibodies and neutralizing antibodies elicited by vaccination or prior infection.
As a result, the new wave of COVID-19 cases driven by the Omicron variant has led to the reinstatement of public health interventions to reduce viral transmission and the reconsideration of current vaccination efforts.
About the study
The researchers of the present study utilized virus-like particles (VLPs) and live viruses to evaluate neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants in 125 fully vaccinated individuals. Blood samples were collected as remnant whole blood and plasma samples from patients hospitalized with COVID-19.
Viral whole-genome sequencing was performed on remnant clinical nasopharyngeal/oropharyngeal (NP/OP) swab samples. The VLPs used in the study incorporated all of the Omicron-specific mutations found in the structural spike, nucleocapsid, matrix, and fusion proteins.
Assembled SARS-CoV-2 genomes were uploaded to the global initiative on sharing all influenza data (GISAID). Raw sequencing data containing genome assembly were simultaneously demultiplexed and converted to FASTQ files and screened for SARS-CoV-2 sequences using a basic local alignment search tool (BLAST).
Study findings
The study findings demonstrated that median VLP neutralizing antibody titers for Delta and Omicron relative to the ancestral wild-type (WT) virus WA-1 lineage in non-boosted vaccinated groups was reduced by 2.7- and 15.4-fold, respectively.
Live virus neutralization titers for both Delta and Omicron were reduced by 3.0 fold. The low reduction for Omicron attributed to the higher limit of detection (LOD) for the live virus with neutralizing antibody titers at 50% inhibition (NT50 = 40) as compared to the VLP neutralization assay that has an NT50 of ten.
About 20% of individuals exhibited neutralizing antibodies to Omicron above an NT50 cut-off of 40 as compared to 80% and 95% for Delta and WT, respectively. In contrast, in the case of the live virus, these percentages were 5%, 45%, and 75% for Omicron, Delta, and WT, respectively.
Baseline VLP titers for WT were 18-fold higher in boosted individuals as compared to non-boosted individuals. There was also a modest reduction in antibody titers at 3.3- and 7.4-fold for Delta and Omicron, respectively, as compared to the WT strain.
Delta breakthrough infections increased median baseline WT neutralization titers by 57-fold and 3.1-fold compared to non-boosted and boosted individuals, respectively. Omicron breakthrough infection resulted in neutralizing antibodies to Omicron in 90% of individuals above an NT50 cut-off of 40, which is comparable to those with neutralizing antibodies to Delta.
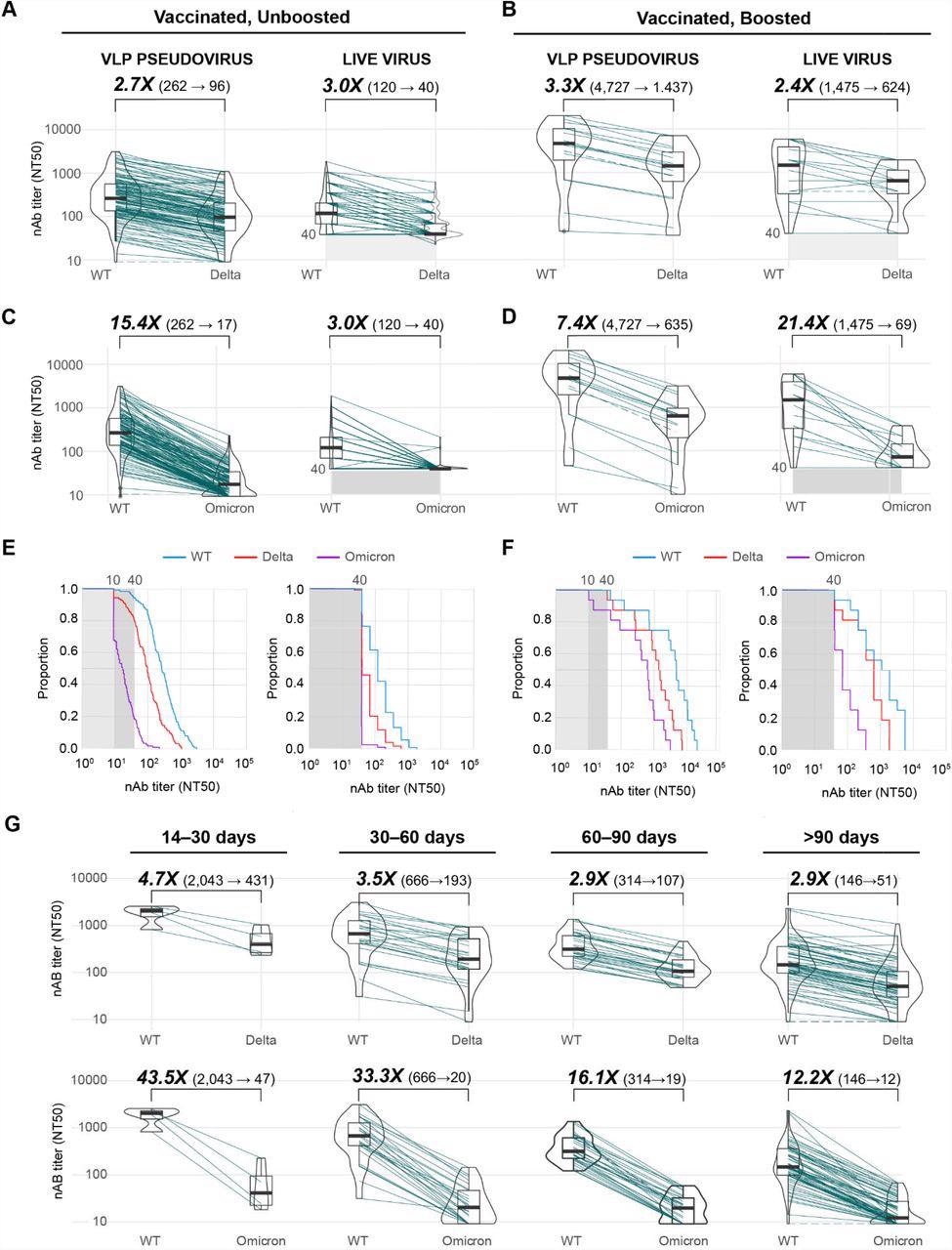
Neutralizing antibody levels in fully vaccinated, uninfected individuals. (A, B) Box-violin plots showing median neutralizing antibody titers using VLP (left) and live virus (right) assays against the SARS-CoV-2 WA-1 ancestral lineage (wild-type, or “WT”) and Delta variant in vaccinated, unboosted (A) and vaccinated, boosted (B) individuals (C, D) Box-violin plots of titers against the WT and Omicron variant in vaccinated, unboosted (C) and vaccinated, boosted (D) individuals. (E, F) Cumulative distribution function plots of titers to WT, Delta, and Omicron using VLP (left) and live virus (right) assays in vaccinated, unboosted (E) and vaccinated, boosted (F) individuals, showing the proportion of samples at or above a given titer. (G) Longitudinal box-violin plots of VLP titers to Delta (top) and Omicron (bottom) stratified by time ranges following completion of a primary vaccine series.
A univariate analysis was performed to identify clinical factors determining neutralizing antibody levels between Delta and Omicron breakthrough infections. The results of the analysis revealed that among various factors analyzed, only clinical severity, COVID-19-related hospitalizations, and a median number of days between the onset of symptoms/polymerase chain reaction (PCR) positivity and sample collection were significant.
There were higher moderate to severe infections among Delta breakthrough cases as compared to Omicron breakthrough cases. Immunocompromised patients with moderate to severe breakthrough infections by Delta and Omicron had higher median levels of neutralizing antibodies in comparison to those with mild or asymptomatic infections.
There was a significant difference in the levels of neutralizing antibodies in Delta and Omicron breakthrough infections, even at comparable time intervals between symptom onset or PCR positivity and specimen collection.
The researchers observed a decrease in the correlation of neutralization and quantitative antibody titers with Omicron and Delta relative to WT. Importantly, it was observed that the majority of Delta breakthrough infection cases with low to moderate levels of spike immunoglobulin G (IgG) antibodies failed to neutralize Omicron in the live virus assay.
Conclusions
The findings of the current study indicate that booster vaccines and breakthrough infections can restore neutralizing hybrid immunity by elevating neutralizing antibody titers, specifically with high titers against infecting variants. Increased neutralizing antibody levels in Delta breakthrough cases compared to Omicron was due to an increased proportion of moderate to severe infections with the Delta variant relative to Omicron infections.
The collective results showed that Omicron-induced immunity may not be sufficient to prevent infection against more pathogenic SARS-CoV-2 variants that are likely to emerge in the future. The researchers highlighted the continued importance of booster vaccines in enhancing immunity against breakthrough infections or future infections from different variants. There is also a need for utilizing variant-specific immunogens in vaccine development as a viable strategy for combating VOCs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Servellita, V., Syed, A. M., Brazer, N., et al. (2022). Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. medRxiv. doi:10.1101/2022.01.25.22269794. https://www.medrxiv.org/content/10.1101/2022.01.25.22269794v1
- Peer reviewed and published scientific report.
Servellita, Venice, Abdullah M. Syed, Mary Kate Morris, Noah Brazer, Prachi Saldhi, Miguel Garcia-Knight, Bharath Sreekumar, et al. 2022. “Neutralizing Immunity in Vaccine Breakthrough Infections from the SARS-CoV-2 Omicron and Delta Variants.” Cell 185 (9): 1539-1548.e5. https://doi.org/10.1016/j.cell.2022.03.019. https://www.cell.com/cell/fulltext/S0092-8674(22)00329-4?.