The coronavirus disease 2019 (COVID-19) pandemic has spread to nearly every country globally, causing over 5.67 million deaths and multiple economic crises, as governments were forced to enact costly and restrictive measures to reduce transmission of the virus.
These measures included mandatory face masks, social distancing guidelines, and even full lockdowns/stay-at-home orders. Many of these were dismantled when vaccines were developed, and mass-administration policies were enacted, but with the emergence and rise to dominance of new variants of concern, case numbers are once again rising, and there are worries over hospital capacity.
Researchers from Moderna, the developers of one key vaccine, have been investigating its ability to protect against the Omicron variant.
Their research can be found on the medRxiv* preprint server while the paper undergoes peer review.
Traditionally, vaccines are created from an inactivated or attenuated form of the virus. This allows the antigens to be presented to the immune system in a controlled environment, making specific antibodies before any potential 'real' infection. While this is effective, there have been safety concerns, as inactivated viruses can become active again, or attenuated strains could receive new traits.
The new messenger RNA (mRNA) vaccines avoid such risks, as no structural proteins are present. In fact, no virus proteins are present in the mRNA vaccines at all. Instead, they contain a small string of mRNA encoding for a particular antigen of the virus and rely on the cells' own machinery to transcribe it. The immune system can become familiarized with the virus with little to no risk.
 Study: Serum Neutralizing Activity of mRNA-1273 Against the SARS-CoV-2 B.1.1.529 (Omicron) Variant: A Preliminary Report. Image Credit: NIAID
Study: Serum Neutralizing Activity of mRNA-1273 Against the SARS-CoV-2 B.1.1.529 (Omicron) Variant: A Preliminary Report. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The Study
The researchers gathered sera from healthy adults enrolled in a phase 2 clinical trial of Moderna's mRNA-1273 COVID-19 vaccine. The sera were collected from 10 matched individuals one month after completing the initial 2-dose vaccination and two weeks following a booster dose. Another ten individuals gave sera only at the two-week post-booster time point.
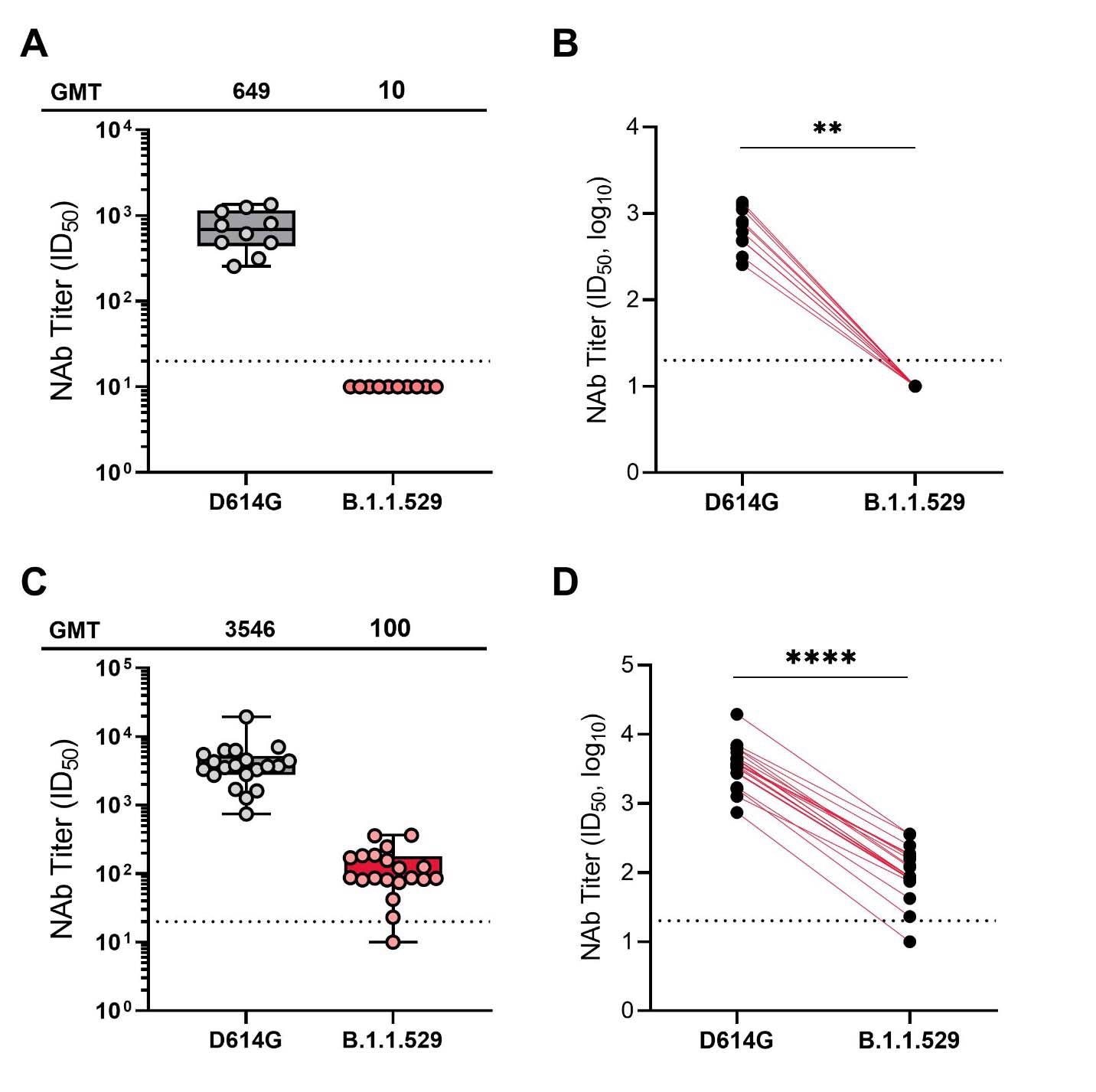 Neutralization of SARS-CoV-2 pseudoviruses in serum samples at 1-month post mRNA-1273 primary series (A and B) and 2 weeks post mRNA-1273 booster (C and D). obtained from participants enrolled in the mRNA-1273 vaccine phase 2 open-label study wherein participants who received a 2-dose primary series (100 μg) during the double-blind phase of the trial received a single booster dose of mRNA-1273 (50 μg). For Panels A and B, samples were collected at Day 57 (1 month following the primary series) and for panels C and D, samples were collected at day 15 (2 weeks following the booster dose). A recombinant vesicular stomatitis virus-based pseudovirus neutralization assay was used to measure neutralization. The pseudoviruses tested incorporated D614G (WT) or the spike substitutions present in B.1.1.529 (Omicron). The reciprocal neutralizing titers on the pseudovirus neutralization assay at ID50 are shown. In panels A and C, boxes and horizontal bars denote the IQR range and the GMT, respectively. Whisker endpoints are equal to the values below or above the median at 1.5 times the IQR. In panels B and D, the colored lines connect the D614G and B.1.1.529 neutralization titers in matched samples. A 2-tailed Wilcoxon matched-pairs signed-rank test was performed (**, P<0.01; ****, P<0.0001). In all panels, the dots represent results from individual serum samples, and the dotted line represents the lower limit of quantification for titers at 20 ID50.
Neutralization of SARS-CoV-2 pseudoviruses in serum samples at 1-month post mRNA-1273 primary series (A and B) and 2 weeks post mRNA-1273 booster (C and D). obtained from participants enrolled in the mRNA-1273 vaccine phase 2 open-label study wherein participants who received a 2-dose primary series (100 μg) during the double-blind phase of the trial received a single booster dose of mRNA-1273 (50 μg). For Panels A and B, samples were collected at Day 57 (1 month following the primary series) and for panels C and D, samples were collected at day 15 (2 weeks following the booster dose). A recombinant vesicular stomatitis virus-based pseudovirus neutralization assay was used to measure neutralization. The pseudoviruses tested incorporated D614G (WT) or the spike substitutions present in B.1.1.529 (Omicron). The reciprocal neutralizing titers on the pseudovirus neutralization assay at ID50 are shown. In panels A and C, boxes and horizontal bars denote the IQR range and the GMT, respectively. Whisker endpoints are equal to the values below or above the median at 1.5 times the IQR. In panels B and D, the colored lines connect the D614G and B.1.1.529 neutralization titers in matched samples. A 2-tailed Wilcoxon matched-pairs signed-rank test was performed (**, P<0.01; ****, P<0.0001). In all panels, the dots represent results from individual serum samples, and the dotted line represents the lower limit of quantification for titers at 20 ID50.
The sera were assessed for their ability to neutralize a recombinant vesicular stomatitis virus pseudotyped with full-length spike protein carrying the D614G mutation, and the B.1.1.529 sublineage – more commonly known as the Omicron variant. A 2-sided Wilcoxon matched-pairs signal rank test was used in order to allow comparison between the wild-type and B.1.1.529 lineage. This included geometric mean titers and a lower limit of quantification.
The researchers found that at one month after the initial set of vaccinations, the neutralizing antibody titers against the Omicron variant were significantly lower than those against wild-type, to the extent that they were below the lower limit of quantification. While the booster did raise the neutralizing titers against Omicron to a more reasonable degree, they were still showed far less neutralizing ability against Omicron than wild-type. The difference was extensive enough that individuals who had not received a booster yet showed higher neutralizing titers against wild-type than boosted individuals showed against Omicron.
The conclusion
While the sample size of this investigation is small, the results are fairly clear. Fully vaccinated individuals, either with or without the booster dose, should show a robust ability to neutralize the wild-type virus but show significantly reduced ability to neutralize the Omicron variant. The booster dose does improve the neutralization titers to the level where severe infection is unlikely, but it seems likely that it would not prevent infection in all individuals.
The authors point out that multiple similar studies have shown that all vaccines appear to have consistently lower performance against the Omicron variant compared to wild-type, but urge individuals to wait until upcoming research with both pseudovirus and live virus neutralization assays is published before coming to the final conclusion, as this could allow more in-depth analysis.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Serum Neutralizing Activity of mRNA-1273 Against the SARS-CoV-2 B.1.1.529 (Omicron) Variant: A Preliminary Report, Diana Lee, Laura E. Avena, Daniela Montes Berrueta, Matthew Koch, Angela Choi, Judy Oestreicher, William Hillebrand, HongHong Zhou, Rolando Pajon, Andrea Carfi, Darin K. Edwards, Kai Wu, medRxiv, 2022.01.28.21268247; doi: https://doi.org/10.1101/2022.01.28.21268247, https://www.medrxiv.org/content/10.1101/2022.01.28.21268247v1
- Peer reviewed and published scientific report.
Choi, Angela, Matthew Koch, Kai Wu, Groves Dixon, Judy Oestreicher, Holly Legault, Guillaume B. E. Stewart-Jones, et al. 2021. “Serum Neutralizing Activity of MRNA-1273 against SARS-CoV-2 Variants.” Edited by Tom Gallagher. Journal of Virology 95 (23). https://doi.org/10.1128/jvi.01313-21. https://journals.asm.org/doi/10.1128/JVI.01313-21.