A new study by Norwegian researchers suggests that vaccine-induced immunological memory and the activation of recall responses in the body yields similar coverage for Omicron and Delta variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The paper is currently available on the medRxiv* preprint server while it undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The SARS-CoV-2 Omicron (B.1.1.529) variant is currently dominating coronavirus disease 2019 (COVID-19) pandemic in many countries around the world. This specific viral variant carries up to 36 mutations in the spike glycoprotein, which is basically the target of vaccine-induced neutralizing antibodies.
Naturally, this raised concerns that standard vaccine dose regimens that are currently offered will not be sufficient in conferring protection and that booster doses are definitely necessary, which was observed as the spread of the Omicron variant intensified.
However, while it seems realistic that Omicron evades immunological memory that is established after vaccination against COVID-19, there is currently little actual data on immune responses to Omicron infection.
In order to appraise re-activation of humoral immunological memory, a research group from the Oslo University Hospital, University of Oslo, and University College of Østfold in Norway measured early induced immune responses in SARS-CoV-2 in double-vaccinated individuals that were infected with Omicron during an outbreak in Oslo in November 2021.
Measuring antibodies and inflammatory mediators
Their methodological approach consisted of measuring antibodies to the SARS-CoV-2 spike glycoprotein and nucleocapsid protein, inflammatory mediators, as well as the release of interferon-gamma in blood.
This was measured in 51 vaccinated individuals infected with Omicron, in 14 infected with Delta, and in 18 healthy controls. Furthermore, two median time points during the first two weeks of infection with the Omicron or Delta variants of SARS-CoV-2 have been used, following the onset of symptoms.
Since the majority of participants have received two doses of mRNA vaccines more than three months prior to infection, the researchers expected that infection with the Delta variant would mimic a booster dose and result in a substantial increase in antibody levels directed against spike glycoprotein.
In the laboratory, Vero E6 cells were used to measure neutralizing antibodies, which is a cell line derived from an African green monkey kidney. Moreover, viral load quantification was also pursued in nasopharyngeal swabs.
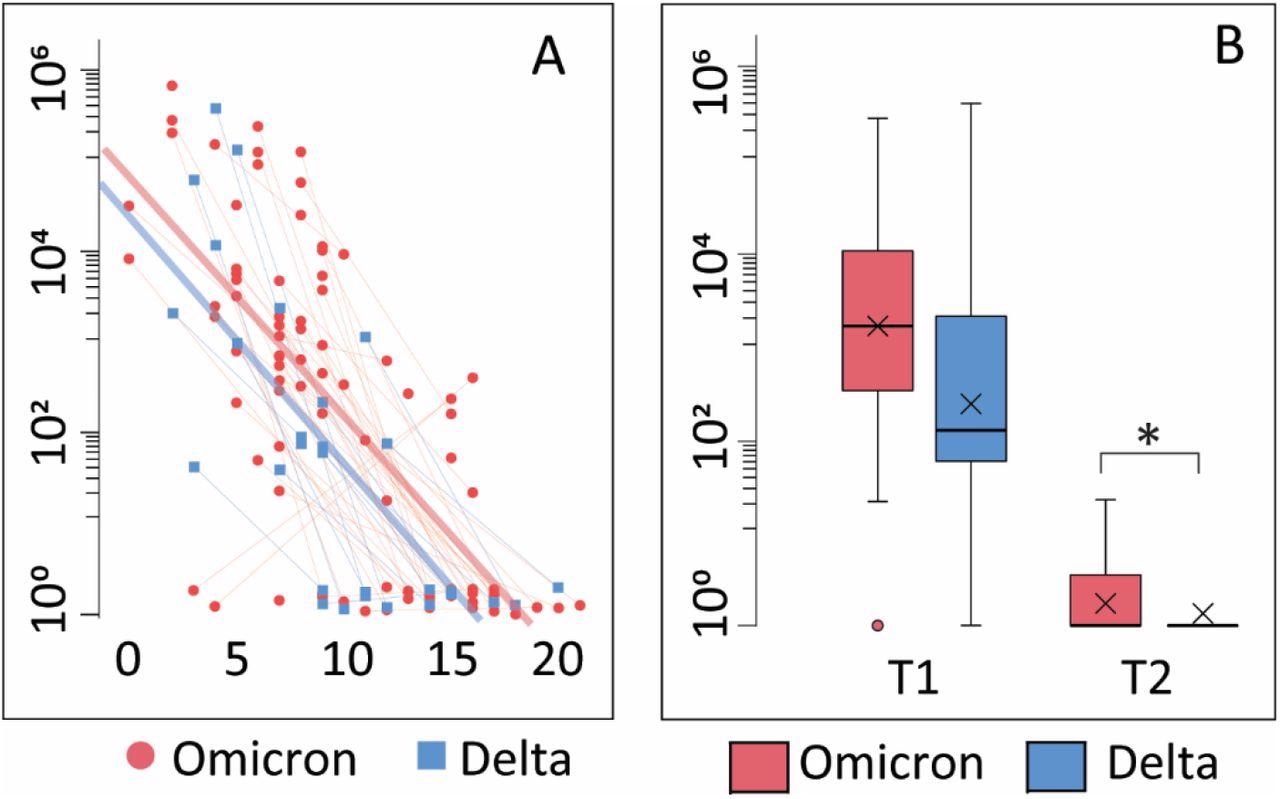
Viral load A: Correlation between viral load and days after symptom onset during the observation period. Thin blue/red lines represent paired samples while thick blue/red lines represent regression curves for the whole group (Omicron or Delta). B: Viral load is shown as Tukey-plots at inclusion (T1) and one-week follow-up (T2) according to infection with Omicron or Delta. *p<0·05 adjusting for symptom days.
Similar coverage for both variants
The results have demonstrated that infection with both Delta and Omicron variants is associated with rather mild symptoms, low levels of inflammatory mediators, and a modest increase in levels of antibodies against the receptor-binding domain (RDB) SARS-CoV-2 spike glycoprotein.
Furthermore, the measurement of interferon-gamma release in the whole blood after stimulation with peptides from the vaccine ancestral SARS-CoV-2 spike glycoprotein highlighted a rapid and similar activation of T-cell mediated immunity after infection with both variants.
In a nutshell, these results suggest that vaccine-induced immunological memory provides similar coverage for the Omicron and Delta SARS-CoV-2 variants in individuals who have received two doses of mRNA COVID-19 vaccine; however, more extended observation periods are necessary for steadfast conclusions on the level of obtained immunity.
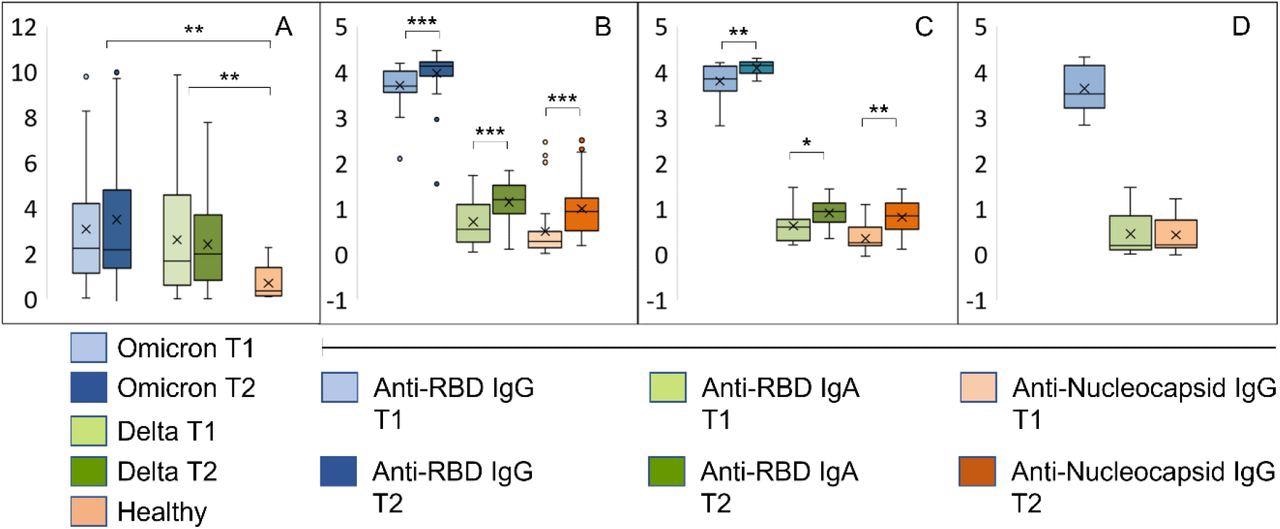
Early immune responses in individuals infected with Omicron or Delta. A: Results from whole blood IFNγ release assay (IGRA, IU/ml) in samples harvested from the same individuals with an interval of 8-10 days. T1: inclusion, T2: one-week follow-up. B-D: The bar graphs show relative levels (log 10) of antibodies to RBD and the nucleocapsid protein in samples described under A. B: Individuals with confirmed Omicron infection, C: Delta infection, D: vaccinated individuals with no history of SARS-CoV-2 infection. ***p<10−5, **p<10−3, *p<10−2..
Putting the results into context
Even though it was expected that infection with the Omicron variant would result in weaker humoral recall responses when compared to the Delta variant, its evasion from humoral immunity may be less severe than indicated from laboratory neutralization assays.
“Due to limitations in the cohort size and observation period, one cannot draw firm conclusions about the exact kinetics of virus clearance,” said study authors in this medRxiv paper. “Yet, in view of the mild symptoms and rapid clinical recovery it seems likely that the combination of circulating antibodies and recall responses in this cohort of double-vaccinated individuals was sufficient for full recovery,” they add.
This is definitely in agreement with the observation that mRNA vaccines elicit highly cross-reactive antibodies to SARS-CoV-2 variants. Still, more studies with even longer follow-up times are needed to reach a robust conclusion on this topic.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Søraas, A., Grødeland, G., Granerud, B.K., et al. (2022). Breakthrough infections with the omicron and delta variants of SARS-CoV-2 result in similar re-activation of vaccine-induced immunity. medRxiv. https://doi.org/10.1101/2022.01.27.22269895, https://www.medrxiv.org/content/10.1101/2022.01.27.22269895v1
- Peer reviewed and published scientific report.
Søraas, Arne, Gunnveig Grødeland, Beathe Kiland Granerud, Thor Ueland, Andreas Lind, Børre Fevang, Sarah L. Murphy, et al. 2022. “Breakthrough Infections with the Omicron and Delta Variants of SARS-CoV-2 Result in Similar Re-Activation of Vaccine-Induced Immunity.” Frontiers in Immunology 13 (September). https://doi.org/10.3389/fimmu.2022.964525. https://www.frontiersin.org/articles/10.3389/fimmu.2022.964525/full.