The coronavirus disease (COVID-19), which arises due to infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causes a wide range of clinical signs and symptoms that can leave the individual asymptomatic or at a high risk of death. Fever, dry cough, dyspnea, weariness, myalgia, anosmia, and ageusia are common signs and symptoms associated with COVID-19.
Older age, concomitant diseases, and possibly, but to a lesser extent, blood type A are all risk factors for poor outcomes of COVID-19. Despite repeated attempts, a specific therapeutic approach to treat COVID-19 has not been established. However, several antiviral drugs like MK-4482/EIDD-2801 and PF-07321332 have recently demonstrated promising results.
Study: High-Dose Convalescent Plasma for Treatment of Severe COVID-19. Image Credit: Kiryl Lis / Shutterstock.com
Background
Viral infections due to Ebola, influenza A, SARS, the Middle East respiratory syndrome (MERS) coronavirus, as well as SARS-CoV-2, have been treated using passive antibody transfer through plasma or serum transfusion. For the treatment of COVID-19, antiviral antibody therapies have been associated with better clinical outcomes when isolated from patient serum or COVID-19 convalescent plasma (CCP). As a result, CCP appears to be an appealing therapy since it could provide neutralizing antibodies.
Recently, an open-label multicenter randomized controlled trial assessed the efficacy and safety of high-dose CCP transfusion in the treatment of severe COVID-19.
Study findings
A total of 36 of the 107 patients recruited for the current study died during their stay in the hospital, with 10 dying after day 30, three of whom were in the CCP group and seven in the control group. On day 30 after randomization, the CCP group had a death rate of 22%, whereas the control group had a death rate of 25%. On day 60, the CCP group had a death rate of 31%, whereas the control group had a death rate of 35%.
The authors also conducted an unscheduled examination of death rates on day 21 after randomization, as the survival curve showed that there might be a difference between the groups. To this end, on day 21, the CCP group had a death rate of 8.3%, while the control group had a death rate of 19.7%.
At randomization on day 30, the CCP and control groups experienced 12.5 and 12 days, respectively, without intrusive mechanical breathing. By day 60, the CCP and control groups had 42.5 and 39 days without intrusive mechanical ventilation.
At days 30 and 60, no changes in hospital stay duration were observed between either group. On day 30, the CCP group had three days of hospital-free time, whereas the control group had 0 days. Furthermore, by day 60, the CCP group had 30.5 days of hospital-free time and the control group had 21.0 days.
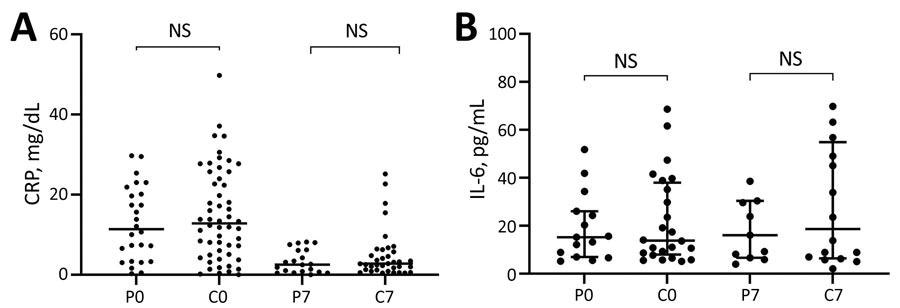
Scatter plots of inflammatory biomarker levels among participants in a study of high-dose convalescent plasma for treatment of severe COVID-19, Brazil. A) C-reactive protein (CRP); total 80 patients (26 CCP, 54 control) on day 0 and 56 (20 CCP, 36 control) on day 7. B) Interleukin-6 (IL-6); total 39 patients (15 CCP, 24 control) on day 0 and 27 (11 CCP, 16 control) on day 7. Horizontal bars indicate medians. C0, control group day 0; C7, control group day 7; COVID-19, coronavirus disease; CCP, COVID-19 convalescent plasma; NS, not significant; P0, convalescent plasma group day 0; P7, convalescent plasma group day 7.
C-reactive protein (CRP) levels were increased at the time of randomization and reduced significantly by day seven in both groups in a similar pattern. On day 0, the median CRP level in the CRP group was 11.4 mg/dL, while the control group had a median CRP level of 12.82 mg/dL. On day seven, the CCP and control groups had median CRP levels of 2.53 mg/dL and 2.75 mg/dL, respectively.
On days 0 and seven, interleukin 6 (IL-6) concentrations were high but did not differ significantly across groups. The median IL-6 levels for the CRP group were 15.20 pg/mL and 13.80 pg/mL on days 0 and seven, respectively, while IL-6 levels for the control group were 16.00 pg/mL and 18.65 pg/mL, respectively.
Implications
The small number of patients included in the current study is one potential limitation; however, because the authors expected difficulties acquiring the required amount of CCP for each patient, they decided to divide the participants into the control and CCP groups.
Additional limitations were that the research was not conducted in a blinded manner and that the administration of a large volume of an intravenous placebo could have been hazardous to the patients. Another limitation was that when the patients got CCP transfusion, they already had SARS-CoV-2 antibodies, which could explain their lack of responsiveness to this therapy.
Taken together, the current study demonstrated that patients with severe COVID-19 received no benefit from high-dose convalescent plasma transfusion. More specifically, patients with severe COVID-19 who received CCP transfusions did not experience any improvement in their death rates at 30 and 60 days after randomization, nor on their time on mechanical ventilation or length of hospital stay.