
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The authors of the current study have previously reported an RBD-scNP vaccine formulation with the 3M-052-aqueous formulation (3M-052-AF) plus Alum that elicited cross-reactive NAbs against SARS-CoV-2 and other sarbecoviruses and conferred protection against the SARS-CoV-2 WA-1 strain in NHPs.
In macaques, RBD-scNPs induced antibodies that neutralized all SARS-CoV-2 variants, including the Beta and Omicron variants, as well as protected against Beta and Delta variant challenges. Moreover, in highly susceptible aged mouse models, RBD-scNP immunization conferred protection against the SARS-CoV-2 Beta variant challenge and against other beta-coronaviruses.
About the study
In the present study, the researchers used RBD-scNPs vaccine formulations to immunize cynomolgus macaques. The macaques were immunized three times four weeks apart. Plasma samples were collected two weeks after the third dose of the RBD-scNP vaccine to assess the neutralizing activity of sera against pseudovirus infection of 293T-ACE2-TMPRSS2 cells by SARS-CoV-2 WA-1 to WA-8 strains.
The researchers also assessed whether the SARS-CoV-2 Omicron variant could escape RBD-scNP-induced NAbs by comparing the neutralizing activity of sera of the D614G and Omicron variants in a 293T-ACE2 pseudovirus assay.
Furthermore, the current study also provides information on whether two doses of RBD-scNP vaccines could protect NHPs from challenge by SARS-CoV-2 WA-1, Beta, or Delta variants. To do this, the researchers immunized macaques with two doses of RBD-scNP vaccines, PBS, or adjuvant alone. Another group of macaques was immunized with soluble RBD to test the effects of multimerization.
Study findings
RBD-scNP induced potent NAbs against the WA-1 strain with a geometric mean titer (GMT) ID50 of 12,266.7, and ID50 titers were reduced further for the other SARS-CoV-2 variants. The researchers noted the highest reduction in neutralizing activity, from 4.1- to 10.2-fold, for the B.1.351 (Beta) variant.
Three doses of RBD-scNP induced serum antibodies that neutralized the D614G and Omicron pseudoviruses, with an observed fold drop of 4.3 in the ID50 GMT and 5.6 in the ID80 GMT. These findings suggest that the RBD-scNP immunization in macaques elicited high titers of NAbs against all SARS-CoV-2 variants, including Omicron.
RBD-scNP vaccination also induced higher NAbs than soluble RBD in five out of eight SARS-CoV-2 variant pseudoviruses. More specifically, the RBD-scNP group exhibited higher titers of NAbs against the WA-1, Alpha, Epsilon, Iota, and Delta viruses.
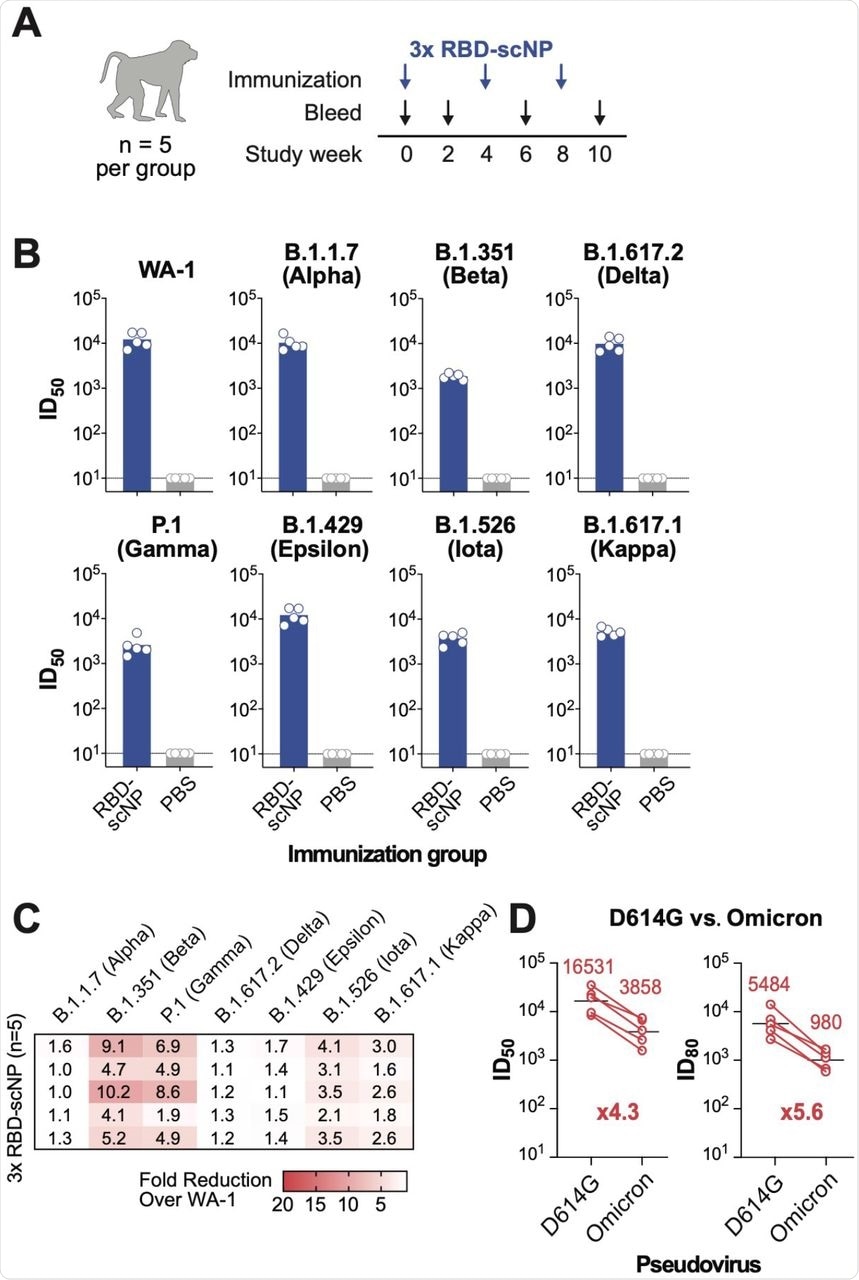
RBD-scNP vaccination elicits broad neutralizing antibodies against SARS-CoV-2 variants.
However, pseudovirus neutralization assays showed comparable neutralizing titers against Beta, Gamma, and Kappa variants by the RBD-scNP, as well as soluble RBD group. Both groups showed decreased neutralizing activity against the Beta, Gamma, and Iota variants as compared to WA-1.
Two weeks after the second vaccination, five macaques from each group were challenged with WA-1, or either the SARS-CoV-2 Beta or Delta variants. On days two and four after the viral challenge, bronchoalveolar lavage (BAL) and nasal swab samples from the PBS or adjuvant group had high copies of the envelope (E) and nucleocapsid (N) gene subgenomic ribonucleic acid (sgRNA). Contrastingly, all five animals in the RBD-scNP group, and four out of the five animals in the soluble RBD group, were fully protected from WA-1 strain, as no sgRNA was detected in their BAL or nasal swab.
After the SARS-CoV-2 Beta variant challenge, nasal N gene sgRNA was detected in only one of the RBD-scNP immunized monkeys but in four of the five immunized monkeys of the soluble RBD group. After the SARS-CoV-2 Delta variant challenge, all animals immunized with two doses of the RBD-scNP vaccine showed no detectable sgRNA in BAL or nasal swab. Post WA-1 and Beta variant challenge, both groups showed no lung tissue hematoxylin and eosin (H&E) staining.
Immunohistochemistry (IHC) staining showed the presence of N antigen in the lungs of macaques who received PBS or adjuvant alone. The N antigen, however, was not detected in the lungs of RBD-scNP or soluble RBD immunized monkeys, thereby indicating that two doses of RBD-scNP protected against viral replication of WA-1, as well as the Beta and Delta variants, in both lower and upper airways. Thus, while lung inflammation was observed in immunized macaques, RBD-scNP was more effective than soluble RBD in protecting against the Beta variant infection in the upper respiratory tract.
The researchers did not observe any enhancement of lung inflammation or virus replication associated with RBD-scNP/Alum formulations. Moreover, the RBD-scNP + 3M-052-AF group was the only group that exhibited reduced severity of lung inflammation, raising the possibility of the use of the Alum alone in protecting monkeys after immunization with RBD-scNPs.
3M-052-AF-adjuvanted vaccine-induced superior systemic and mucosal antibody responses and higher titers of NAbs than 3M-052-AF + Alum-adjuvanted vaccine, thus demonstrating that 3M-052-AF in the absence of Alum is an optimal adjuvant for scNP.
Conclusions
Taken together, the study results demonstrate that scNP vaccines confer adequate protection in NHPs against the WA-1, Beta, and Delta variants by inducing NAbs against all the SARS-CoV-2 variants tested in vitro. These findings could prove useful during the development of the second-generation COVID-19 vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Li, D., Martinez, D. R., Scafer, A., et al. (2022). Breadth of SARS-CoV-2 Neutralization and Protection Induced by a Nanoparticle Vaccine. bioRxiv. doi:10.1101/2022.01.26.477915. https://www.biorxiv.org/content/10.1101/2022.01.26.477915v1.
- Peer reviewed and published scientific report.
Li, Dapeng, David R. Martinez, Alexandra Schäfer, Haiyan Chen, Maggie Barr, Laura L. Sutherland, Esther Lee, et al. 2022. “Breadth of SARS-CoV-2 Neutralization and Protection Induced by a Nanoparticle Vaccine.” Nature Communications 13 (1): 6309. https://doi.org/10.1038/s41467-022-33985-4. https://www.nature.com/articles/s41467-022-33985-4.