The emergence of multiple severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants possessing mutations in the spike RBD raised the concern for neutralizing antibody evasion. Beta and Omicron variants have a greater immune escape ability than the Alpha and Delta variants. Epidemiological evidence also suggested decreasing SARS-CoV-2 vaccine effectiveness against the Omicron variant.
The current research work focused on measuring the memory B cell breadth against the Beta and Omicron variants and the temporal shift in the memory B cell subset composition over time following two doses of an mRNA vaccine.
Study: SARS-CoV-2 Omicron-neutralizing memory B-cells are elicited by two doses of BNT162b2 mRNA vaccine. Image Credit: Kateryna Kon/ Shutterstock
Study design
The study protocol involved the participation of 45 healthcare workers post receiving two doses of the mRNA vaccine BNT162b2 (Pfizer-BioNTech). The circulating antibodies and memory B cells in the peripheral blood at the early (31 days) and late (146.5 days) time points after the second vaccine dose were measured. The concentration of RBD binding immunoglobulin G (IgG) and IgA antibodies titers in plasma was quantified by electrochemiluminescence immunoassay (ECLIA). The neutralizing antibody titers against the Beta and Delta variants were determined by the microneutralization test (MNT) and that against the Omicron variant by the pseudovirus neutralization test (PV-NT) assay.
The neutralizing breadth of antibodies against the variants was assessed by the neutralization breadth index (NBI) that represented the relative neutralizing activities to the Wuhan strain versus those to the variants. Flow cytometry was used for measuring frequencies and phenotypes of RBD binding memory B cells in the peripheral blood. Researchers prepared recombinant Omicron RBD protein for quantifying the broad reactivity of memory B cells against the variants.
Findings
The researchers observed after one month that two doses of the mRNA vaccine strongly increased Wuhan RBD IgG titers to more than 2000-folds compared to the pre-vaccination level. Beta variant neutralizing antibodies decreased to a lesser extent compared to Wuhan and Delta, while Omicron variant neutralizing antibodies were highly reduced compared to other variants. NBI was increased to two-fold, and 2.8 fold against the Beta variant but no increase was shown against the Delta variant.
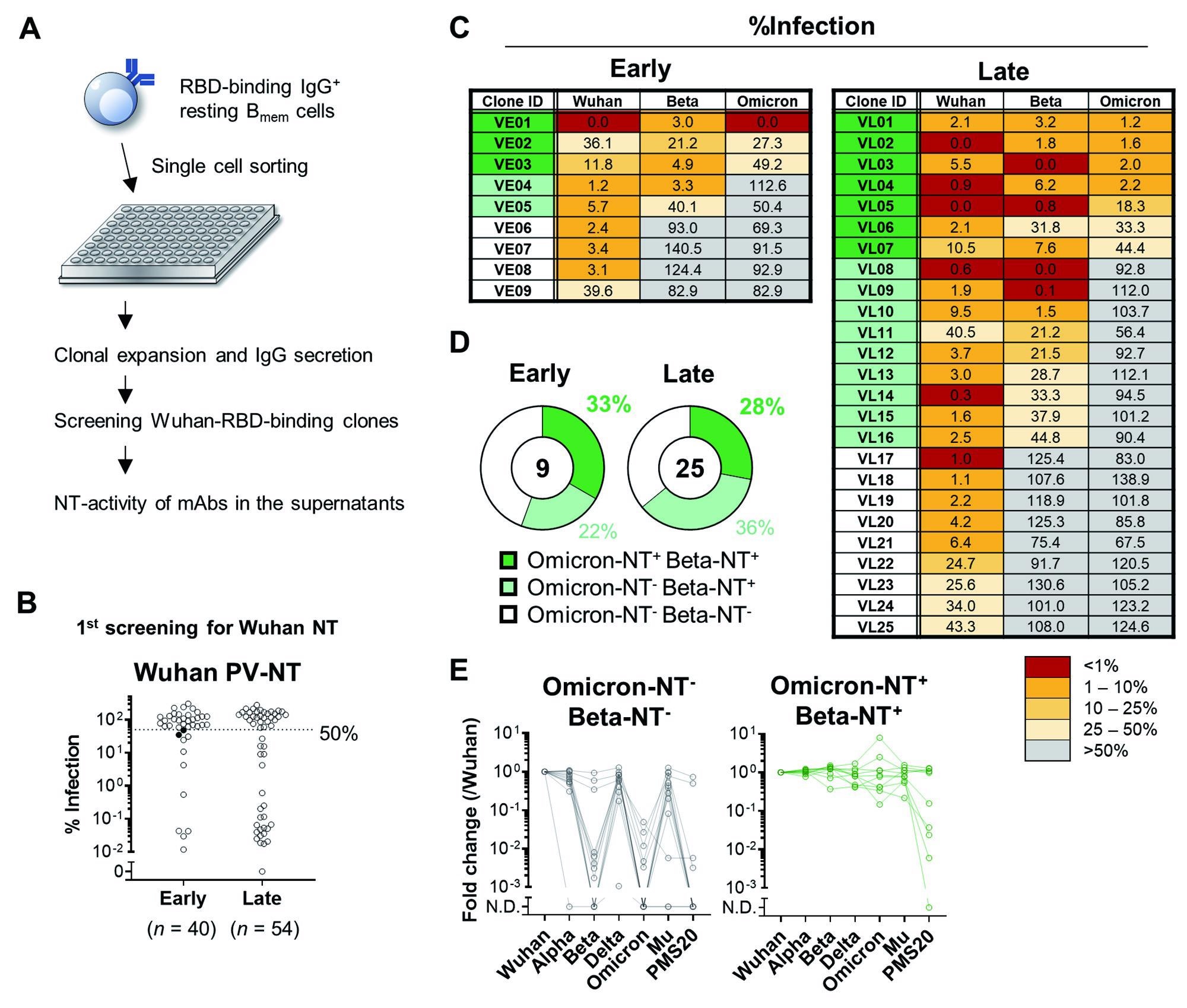 The broadly-neutralizing activity of Bmem-derived monoclonal antibody.(A) Schematic diagram of the experimental workflow for assessing the neutralizing (NT) activity of monoclonal antibodies expressed by Bmem cells. In total, 229 monoclonal B cells were expanded and secreted >10 ng/mL IgG in the supernatants. Of those, 94 clones bound the Wuhan RBD in ELISA and were further tested for NT activities to the Wuhan pseudovirus. (B) NT activities of individual monoclonal antibodies at the first screening were plotted as percent infection calculated as the signals detected by individual antibodies out of those without antibodies. The antibodies with less than 50% of values were considered positive for neutralizing activities. The two filled dots were excluded as the mean of three independent experiments exceeded 50% of the cut-off value. (C) Mean % infection of monoclonal antibody clones from early (n = 9) and late (n = 25) time points of three independent experiments were indicated. (D) Pie charts represent the ratios of monoclonal antibody clones with Beta- and Omicron-neutralizing activity. (E) The binding breadth of monoclonal antibody clones listed in (C) was quantified with ECLIA. Signals to each RBD were normalized to those of reference antibody and fold changes to Wuhan RBD were calculated. Clones neutralizing Wuhan-only (Omicron-NT- Beta-NT-, n = 13) and Wuhan/Beta/Omicron (Omicron-NT+ Beta-NT+, n = 10) were plotted separately.
The broadly-neutralizing activity of Bmem-derived monoclonal antibody.(A) Schematic diagram of the experimental workflow for assessing the neutralizing (NT) activity of monoclonal antibodies expressed by Bmem cells. In total, 229 monoclonal B cells were expanded and secreted >10 ng/mL IgG in the supernatants. Of those, 94 clones bound the Wuhan RBD in ELISA and were further tested for NT activities to the Wuhan pseudovirus. (B) NT activities of individual monoclonal antibodies at the first screening were plotted as percent infection calculated as the signals detected by individual antibodies out of those without antibodies. The antibodies with less than 50% of values were considered positive for neutralizing activities. The two filled dots were excluded as the mean of three independent experiments exceeded 50% of the cut-off value. (C) Mean % infection of monoclonal antibody clones from early (n = 9) and late (n = 25) time points of three independent experiments were indicated. (D) Pie charts represent the ratios of monoclonal antibody clones with Beta- and Omicron-neutralizing activity. (E) The binding breadth of monoclonal antibody clones listed in (C) was quantified with ECLIA. Signals to each RBD were normalized to those of reference antibody and fold changes to Wuhan RBD were calculated. Clones neutralizing Wuhan-only (Omicron-NT- Beta-NT-, n = 13) and Wuhan/Beta/Omicron (Omicron-NT+ Beta-NT+, n = 10) were plotted separately.
The researchers observed a reduction in IgG antibodies in the vaccinated individual, while there was an increase in the level of RBD-binding IgG+ B cells to 1.8 fold since early to late time points compared to IgA+ and IgM+ B cells, which were less persistent. CD27+ gating decreased numbers of IgM+ B cells to 2.4 to 3.2-fold compared to those before the gating; however, the IgM+ cells were less prominent than IgG+ cells.
At the early time points, in the vaccinated groups, there was an induction of four subsets in the RBD-binding IgG+ memory B cells. However, after 4.9 months of vaccination, there was a 3.5-fold increase in the number of resting memory B subsets and a decrease in atypical memory B subsets. Irrespective of RBD-binders or non-RBD-binders, IgG+ memory B cells composition bound to spike protein modulated over time, and the resting memory B subset was the dominant subset at late time points.
The study results showed that the majority of IgG+ memory B cells bound to RBD were reactive against the RBD variants of Beta and Delta. At early time points, Omicron reactivity was also observed in these cells which increased from 38% to 52% in the resting memory B subsets.
IgG derived from memory B cells retained the neutralizing effect against Beta and Omicron variants.
All the Wuhan-specific neutralizing clones poorly bind with the Omicron RBD but positively associate with Alpha, Delta, and Mu variants. The majority of these clones did not bind to Beta RBD except for three clones - VE08, VE09, and VL22. Two clones - VE08 and VE09 - bound polymutant spike protein 20 (PMS20) and reflected the broad RBD binding of these clones but not for Omicron RBD. Moreover, at least half of the Omicron-specific neutralizing clones showed high affinity to PMS20.
Conclusion
The current study results showed that two doses of the mRNA vaccine-elicited plasma neutralizing antibodies with limited activity against Beta and Omicron but induced an expanded antibody breadth over time. While more than one-third of RBD-binding IgG+ memory B cells with a resting phenotype initially bound the Beta and Omicron variants and steadily increased the B cell receptor (BCR) breadth over time. Resting memory B cells subset secreted Beta- and Omicron-neutralizing antibodies when stimulated in vitro.
Taken together, the findings supported the potential role of vaccine-elicited memory B cell subsets to the neutralizing breadth of recall antibodies following the third mRNA vaccine dose or non-Omicron breakthrough infection.
Journal reference:
- SARS-CoV-2 Omicron-neutralizing memory B-cells are elicited by two doses of BNT162b2 mRNA vaccine. Ryutaro Kotaki, Yu Adachi, Saya Moriyama, Taishi Onodera, Shuetsu Fukushi, Takaki Nagakura , Keisuke Tonouchi , Kazutaka Terahara, Lin Sun, Tomohiro Takano, Ayae Nishiyama, Masaharu Shinkai, Kunihiro Oba, Fukumi Nakamura-Uchiyama, Hidefumi Shimizu, Tadaki Suzuki , Takayuki Matsumura , Masanori Isogawa,Yoshimasa Takahashi. 2022. Science Immunology, DOI: 10.1126/sciimmunol.abn8590, https://www.science.org/doi/10.1126/sciimmunol.abn8590