Antiviral therapy holds promise in the secondary prevention of coronavirus disease 2019 (COVID-19) by preventing disease progression and thus reducing the severity of illness and the burden on healthcare facilities. The best time for such treatment is in early infection, preventing viral replication and lowering the viral load. A new preprint research paper examines the efficacy of using three antivirals in various combinations in early infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
Antiviral chemotherapy should preferably be carried out with multiple agents that act via different mechanisms of action, such as the sequential interruption of replication pathways using a polymerase inhibitor with a protease inhibitor. The prior outbreak of SARS-CoV led to the use of ribavirin and lopinavir-ritonavir, which belong to these two drug classes, respectively.
The effect was a reduction in mortality and intubation requirements when the combination was used early. Conversely, the late treatment showed little utility.
With the currently circulating SARS-CoV-2, ribavirin was not useful, but in vitro potency was demonstrated with favipiravir, an oral polymerase inhibitor. In addition, animal studies have shown viral load reduction and less lung pathology at high favipiravir doses, and early human studies also suggested some benefits, though this is controversial.
Lopinaivir-ritonavir is less potent against this virus in vitro than other anti-HIV drugs, but their better safety profile and more extended experience led to their use in the current study, which is published on the medRxiv* preprint server. They are expected to reduce viral replication by about 30% at the approved dose, which, as predicted, did not lead to better clinical outcomes in hospitalized patients.
As a result, the FLARE trial aimed at using these less potent drugs at an earlier stage in viral replication in a phase 2 trial of these drugs. These three drugs were used in a 2x2 factorial design, comparing monotherapy with combinations, all against placebo. This ensured that the combination of each drug was precise while enhancing or unfavorable effects were also plain.
The doses used were decided according to available safety data, and 90% effectiveness concentrations (EC90) were determined according to modeling based on available pharmacokinetics.
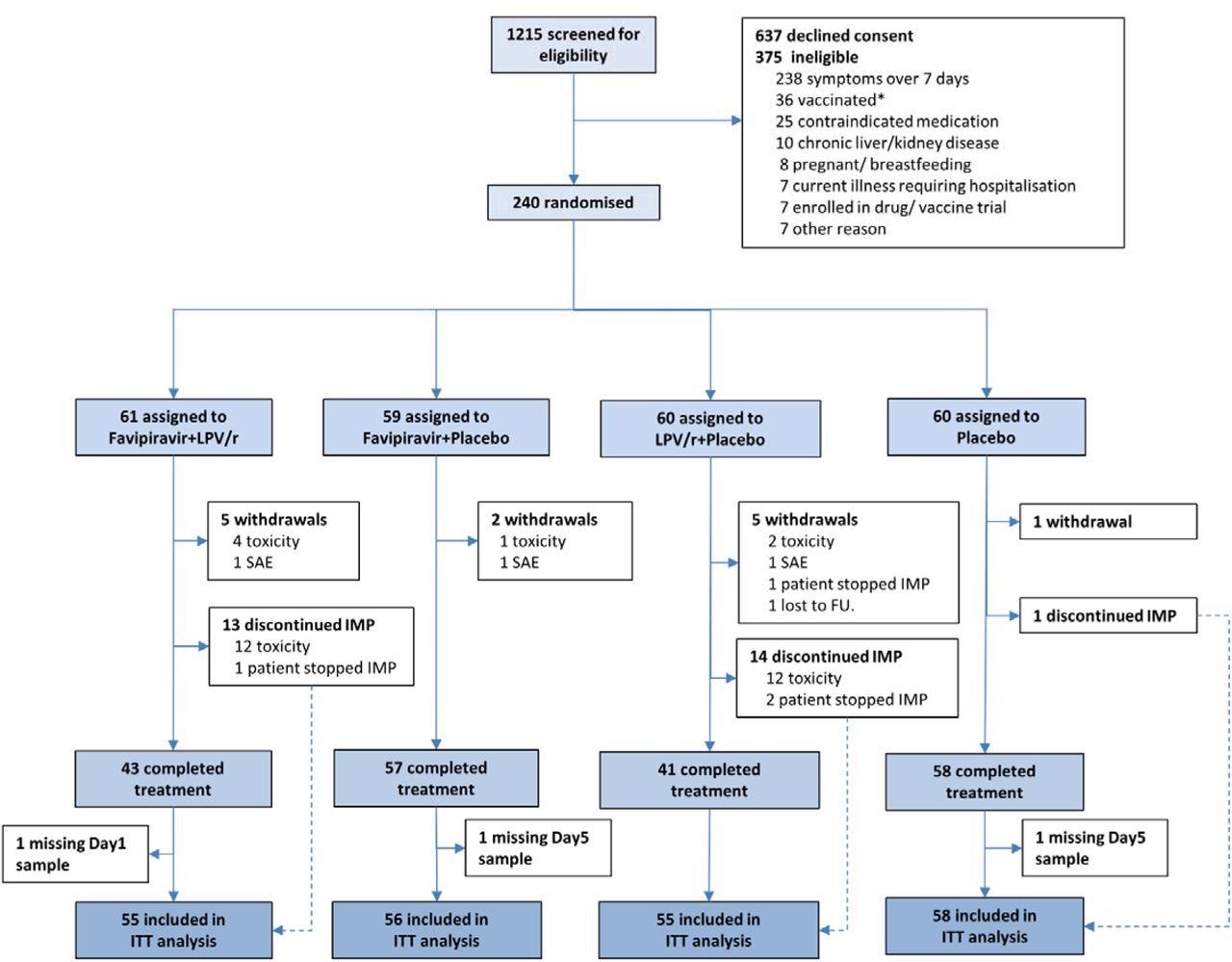
CONSORT diagram for the FLARE trial. * SARS-CoV-2 vaccination was an exclusion in the earlier part of the trial.
What Did the Study Show?
The study included 224 subjects, mostly healthy Caucasians without underlying illnesses, below the age of 55 years. Half of them were vaccinated. Two of every three were seropositive at baseline, and the same proportion began treatment within five days of symptoms.
The study groups included favipiravir plus lopinavir-ritonavir, favipiravir plus lopinavir-ritonavir placebo, favipiravir placebo plus lopinavir-ritonavir, or placebos of both drugs.
On day 5, there was no significant reduction in viral load in any group, nor was there any significant interaction between the drugs used in any combination. However, with favipiravir monotherapy, there was a reduction of -0.65 log10 copies/mL. The effect was most significant in patients with a higher viral load at the time of favipiravir treatment, with the viral load being 1.30 log10 copies/mL lower compared to placebo.
This group also had a higher risk of being virus-negative by Day 5, the odds being 2.5 times higher, but not any of the other groups. Vaccination or antibody status, or time since symptom onset, did not show any effect on treatment effect.
However, when the mean viral load in the intention-to-treat (ITT) population was plotted daily against the proportion with undetectable viral loads, vaccinated or seropositive subjects showed the highest reduction in viral load. The presence of antibodies was also correlated with slightly reduced baseline viral loads in all treatment groups.
Patients receiving lopinavir-ritonavir monotherapy and all three drugs were more likely to have adverse effects, reported in approximately 90% of participants in either, versus less than half in the favipiravir group, and less than a third in the placebo group. The risk in the first group was 16-fold higher compared to placebo.
Especially, diarrhea and nausea were present in both these treatment groups. More participants discontinued treatment in the lopinavir-ritonavir (mono- or multi-therapy) groups. Serum uric acid levels went up with favipiravir treatment, with a 19-fold higher risk in the monotherapy group at day 7. However, full-resolution was seen on day 14.
One serious adverse event occurred in each of the drug treatment groups, and one patient in the favipiravir monotherapy group required intensive care.
Evidence of a new drug interaction resulting in drug antagonism was seen between favipiravir and lopinavir-ritonavir, with lower peak and trough favipiravir levels being observed in the combination group versus the monotherapy group. Most patients did not achieve the EC90 dose.
Fever was uncommon, and the humoral response in all groups was comparable.
What Are the Implications?
“The major finding of FLARE is that, at the doses used, there is no clear evidence that either favipiravir monotherapy or favipiravir plus lopinavir-ritonavir produce clinically worthwhile reductions in viral load in early treatment.”
However, there was a trend towards a reduction in viral load with favipiravir monotherapy, and this group also had more patients with an undetectable viral load – especially when the baseline viral load was high.
In explanation, it appears that patients with a low viral load already have slowing viral replication. Hence, the antivirals are unlikely to reduce it anymore and suggesting the patient subset likely to benefit the most.
Favipiravir shows a similar magnitude of difference to molnupiravir, which acts the same way and demonstrated clinical effectiveness in early COVID-19. However, it has not yet come into routine clinical use.
Further studies are required to understand why favipiravir levels did not reach the expected concentrations, but possible reasons could be the gut symptoms with lopinavir-ritonavir combination, or because more doses were missed but not reported in the combination group.
The study does rule out the clinical utility of lopinavir-ritonavir in early infection, showing that it neither reduces the viral load nor is it well tolerated. Cogently, the researchers remark: “We were able to reach this conclusion by exposing only 60 outpatients to lopinavir-ritonavir monotherapy. A similar design could have quickly ruled out other repurposed agents such as hydroxychloroquine.”
Other combinations, including ritonavir must be screened carefully for adverse effects, especially since the lopinavir-ritonavir combination shows frequent side effects and adverse interactions with other commonly used medications.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Lowe, D. M. et al. (2022). Favipiravir, Lopinavir-Ritonavir or Combination Therapy (FLARE): A Randomised, Double Blind, 2x2 Factorial Placebo-Controlled Trial Of Early Antiviral Therapy In COVID-19. medRxiv preprint. doi: https://doi.org/10.1101/2022.02.11.22270775. https://www.medrxiv.org/content/10.1101/2022.02.11.22270775v1
- Peer reviewed and published scientific report.
Lowe, David M., Li-An K. Brown, Kashfia Chowdhury, Stephanie Davey, Philip Yee, Felicia Ikeji, Amalia Ndoutoumou, et al. 2022. “Favipiravir, Lopinavir-Ritonavir, or Combination Therapy (FLARE): A Randomised, Double-Blind, 2 × 2 Factorial Placebo-Controlled Trial of Early Antiviral Therapy in COVID-19.” PLOS Medicine 19 (10): e1004120. https://doi.org/10.1371/journal.pmed.1004120. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1004120.