New research in nursing home residents found that a third dose of the Pfizer-BioNTech COVID-19 vaccine was effective for at least three months in protecting against the Delta and Omicron variant. While two doses of an mRNA COVID-19 vaccine showed poor neutralization to Omicron than Delta, a booster shot increased neutralizing antibody responses.
Older adults over 65 and people who are immunocompromised have a higher risk of developing severe COVID-19 infection. The emergence of the Omicron variant further increases the risk as it is more transmissible than Delta and has partial immune evasion to two-dose vaccines.
The findings suggest repeated COVID-19 doses are a viable option in strengthening the immune defense of high-risk populations. “We can hypothesize that the combined effect of low but adequate immunization, and the lower risk of complications reported in vaccinated healthy subjects, may contribute to a lower risk of severe Omicron infection in boosted older people,” wrote the research team.
The study, “Immunogenicity of a BNT162b2 vaccine booster against SARS-CoV-2 Delta and Omicron variants in older people”, is currently available on the Preprints with The Lancet* server.
 Study: Immunogenicity of a BNT162b2 Vaccine Booster Against SARS-CoV-2 Delta and Omicron Variants in Older People. Image Credit: Skylines / Shutterstock
Study: Immunogenicity of a BNT162b2 Vaccine Booster Against SARS-CoV-2 Delta and Omicron Variants in Older People. Image Credit: Skylines / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Patient characteristics and study details
The study took place in Lille University Hospital in France, where the study authors recruited 106 nursing home residents over 65 who were vaccinated with both doses of the Pfizer-BioNTech COVID-19 vaccine. None of the study participants had a current or persistent infectious disease, a neoplasia diagnosis in the last five years, or were actively taking steroids and/or immunosuppressants. The team also collected information on past COVID-19 infections or high antibody levels for the SARS-CoV-2 spike protein S1 domain.
Of the 106 residents, 47 had no history of COVID-19 infection before vaccination, and 59 had a COVID-19 diagnosis before any vaccination. Unfortunately, six participants died before collecting antibody data after the booster shot, and data on 11 residents were not available at the time of analysis.
A total of 87 nursing home residents donated serum samples three months after the third booster dose. The researchers measured IgG levels in serum samples and how these antibodies fared when exposed to either the Delta or Omicron strains.
Immune levels before and after vaccination
Before vaccination, nursing home residents who had no prior exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had lower IgG levels that targeted the spike protein S1/S2 domain compared to residents who had previously recovered from illness. After the booster dose, both groups showed high antibody levels.
The T cell count was also higher in nursing home residents who had a history of SARS-CoV-2 infections compared to those never exposed. After the two-dose vaccination, researchers observed T cell responses remained stable for up to eight months. The Pfizer-BioNTech booster increased the number of S1 reactive T cells in both groups a month after the booster dose.
Neutralizing antibody response against Delta and Omicron after booster
Before the booster, about 19% of nursing home residents with no prior COVID-19 illness had neutralizing antibodies against the Delta variant. However, having a previous infection helped; 88% of nursing home residents showed antibodies for Delta.
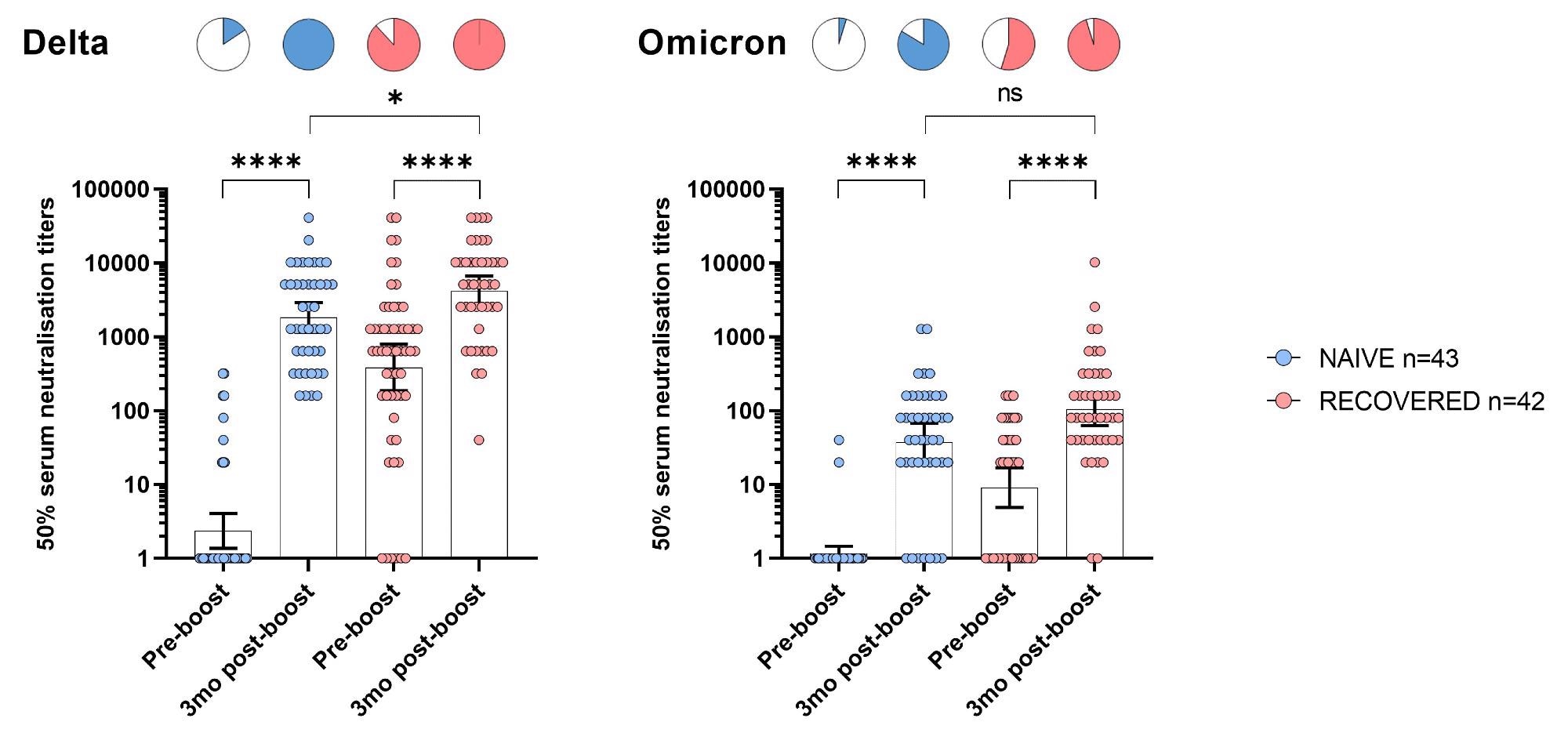 Neutralization of the Delta and Omicron variants before and 3 months after booster BNT162b mRNA vaccine dose in NH residents.
Neutralization of the Delta and Omicron variants before and 3 months after booster BNT162b mRNA vaccine dose in NH residents.
Three months after a booster shot, all nursing home residents in the study showed detectable and high levels of neutralizing antibodies against the Delta variant. Moreover, nursing home residents who were both boosted and recovered from infection had significantly higher antibody titers than the COVID-naive group.
In part, Omicron was less sensitive to neutralizing antibodies because of mutations that allow it to evade the immune system. Against Omicron but before the booster, only 5% of the COVID-19 naive group had detectable antibody levels. In contrast, 55% of residents who recovered from infection had antibodies against Omicron.
When researchers measured antibody levels three months after receiving a Pfizer-BioNTech booster, 84% of nursing home residents had detectable neutralizing antibodies against Omicron. In addition, about 95% of residents who recovered from infection had antibodies. However, there was a 34- to 35-fold reduction in neutralizing titers against Omicron compared to Delta.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Alidjinou EK, et al. (2022). Immunogenicity of a BNT162b2 Vaccine Booster Against SARS-CoV-2 Delta and Omicron Variants in Older People. SSRN First Look. DOI: http://dx.doi.org/10.2139/ssrn.4037259, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4037259
- Peer reviewed and published scientific report.
Alidjinou, Enagnon Kazali, Julie Demaret, Bénédicte Corroyer-Simovic, Julien Labreuche, Anne Goffard, Jacques Trauet, Daniela Lupau, et al. 2022. “Immunogenicity of BNT162b2 Vaccine Booster against SARS-CoV-2 Delta and Omicron Variants in Nursing Home Residents: A Prospective Observational Study in Older Adults Aged from 68 to 98 Years.” The Lancet Regional Health - Europe 17 (June): 100385. https://doi.org/10.1016/j.lanepe.2022.100385. https://www.sciencedirect.com/science/article/pii/S2666776222000783.