
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 has resulted in more than 436 million cases and over 5.95 million deaths globally to date, leading to substantial socio-economic repercussions. The active transmission of SARS-CoV-2 has led to the emergence of heavily mutated SARS-CoV-2 VOC like Omicron with high transmissibility, which poses a challenge to public health management methods to limit the COVID-19 pandemic. Furthermore, apart from high transmissibility, the recently emerged Omicron variant exhibits substantial immune evasion from neutralizing responses induced by COVID-19 vaccinations or natural infections relative to the previous SARS-CoV-2 VOCs.
Several potent monoclonal Abs (mAbs) have been given emergency approval by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the SARS-CoV-2 treatment. Nonetheless, almost all of these mAbs were ineffective against the SARS-CoV-2 Omicron variant due to its high number of mutations compared to the existing variants.
FBR-002 consists of purified polyclonal equine fragments with two antigen-binding regions (F(ab’)2) targeting the SARS-CoV-2 spike (S) protein and is considered an efficient and safe therapy against SARS-CoV-2 compared to polyclonal humanized anti-SARS-CoV-2 antibodies or convalescent plasma therapy. However, in-depth information about FBR-002 is not yet available.
About the study
In the present research, the scientists performed an in vitro assessment of a clinical-grade product of polyclonal F(ab’)2 immunoglobulin (Ig) fragments from Fab’entech, named FBR-002 against the SARS-CoV-2 VOCs. Equine hyperimmune plasma was procured following the hyper immunization of three healthy French trotter horses with SARS-CoV-2 S protein. The purification of pooled horse plasma through 1) anion-exchange chromatography, 2) hydrolysis of whole Igs, and 3) pasteurization resulted in highly purified F(ab’)2 fragments.
The team evaluated the activity of FBR-002 against SARS-CoV-1, Middle East respiratory syndrome CoV (MERS-CoV), and SARS-CoV-2 D614G, Alpha, Beta, Gamma, Delta, Omicron, Mu, Kappa, Iota, and Epsilon variants using baby hamster kidney cells (BHK-21) and African Green Monkey Cell Line (VeroE6). The neutralizing activity of FBR-002 was determined using a SARS-CoV-2-pseudotyped recombinant vesicular stomatitis virus-luciferase (rVSV-luc) system. Additionally, the rVSV-luc pseudotypes of VSV and Ebola virus (EBOV) served as controls.
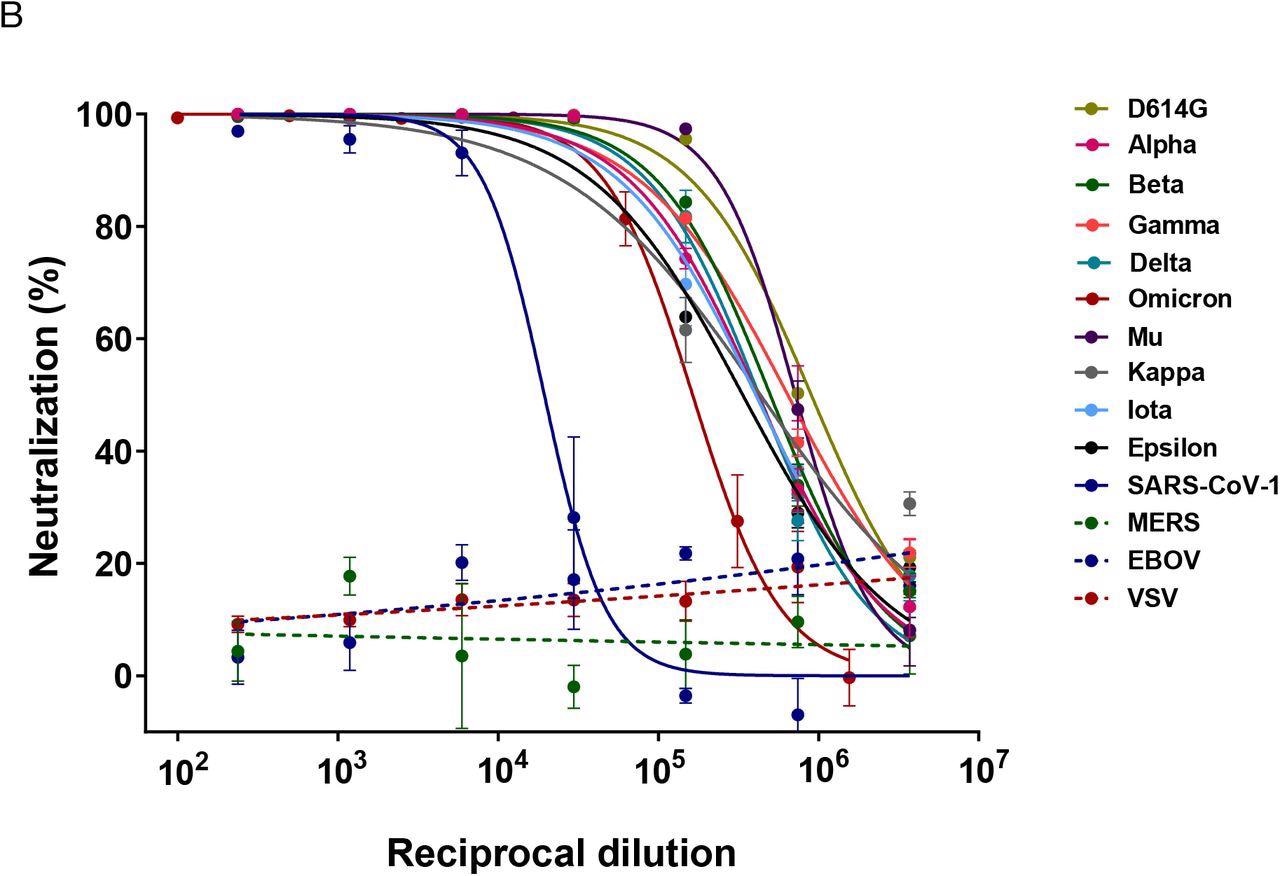
SARS-CoV-2 neutralizing activity curves for FBR-002 against SARS-CoV-2 VoC: reference D614G, Alpha, Beta, Gamma, Delta, Omicron, Mu, Kappa, Iota, Epsilon, SARS-CoV-1, MERS, EBOV and VSV. Neutralizing activity curves are presented using nonlinear regression model fit with settings for log inhibitor vs normalized response curve by GraphPad Prism V8.
Study findings
The results demonstrate that the FBR-002 has significant neutralization potency against the SARS-CoV-2 variants evaluated. Neutralization titers of FBR-002 against all SARS-CoV-2 variants tested varied between 105 and 106 IU/mL and reached more than 200,000 IU/mL for most SARS-CoV-2 VOCs assessed.
The highest reduction in neutralizing titers of FBR-002 was against the SARS-CoV-2 Omicron variant relative to the SARS-CoV-2 ancestral D614G sequence. However, the neutralization titer of FBR-002 against Omicron was 111,403 UI/mL, which was two-to-three orders of magnitude higher than that generally achieved by the SARS-CoV-2 booster vaccination or vaccinating COVID-19-recovered individuals. These inferences were probably due to the presence of high titers of a repertoire of antibodies directed towards conserved epitopes in diverse locations of the SARS-CoV-2 S protein.
Nevertheless, the neutralization titer of FBR-002 against SARS-CoV-1 was much lower than SARS-CoV-2 and was at 13,232 IU/mL. Further, FBR-002 exhibited no measurable neutralization potency against the EBOV, VSV, or MERS-CoV.
Conclusions
The study findings suggest that the FBR-002 exhibited substantial neutralization potency against all the SARS-CoV-2 variants assessed, including the Omicron. The exceptionally high neutralization breadth of FBR-002 observed in the study was probably because of the high titers of a repertoire of antibodies aiming at conserved epitopes in various sites of the SARS-CoV-2 S, such as the receptor-binding domain (RBD) and N-terminal domain (NTD).
The study indicates that potency and breadth are two significant attributes of a high-titer polyclonal preparation that might be very useful in targeting an evolving agent like SARS-CoV-2. This observation is of prime significance in light of the high transmission levels of the heavily mutated variants like Omicron, even in highly vaccinated populations.
Besides, FBR-002 also demonstrated neutralizing activity against SARS-CoV-1, responsible for the SARS epidemic of 2002-2003, although the activity was lower than that against the SARS-CoV-2. In short, the study supports the development of anti-SARS-CoV-2 equine polyclonal F(ab')2 antibodies as a new therapeutic approach against COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Potent Neutralizing Activity of Polyclonal Equine Antibodies against Omicron SARS-CoV-2, Joanna Luczkowiak, Pauline Radreau, Ludovic Nguyen, Nuria Labiod, Fatima Lasala, Cecile Helene Herbreteau, Rafael Delgado, bioRxiv, 2022.02.21.481341; doi: https://doi.org/10.1101/2022.02.21.481341, https://www.biorxiv.org/content/10.1101/2022.02.21.481341v2
- Peer reviewed and published scientific report.
Luczkowiak, Joanna, Pauline Radreau, Ludovic Nguyen, Nuria Labiod, Fátima Lasala, Francisco Veas, Cécile Hélène Herbreteau, and Rafael Delgado. 2022. “Potent Neutralizing Activity of Polyclonal Equine Antibodies against Severe Acute Respiratory Syndrome Coronavirus 2 Variants of Concern.” The Journal of Infectious Diseases 227 (1): 35–39. https://doi.org/10.1093/infdis/jiac331. https://academic.oup.com/jid/article/227/1/35/6654838.