The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) has led to the worst pandemic of the century. The high cumulative mortality of the coronavirus disease 2019 (COVID-19) is primarily due to life-endangering complications such as acute respiratory distress syndrome (ARDS) and systemic hyperinflammation causing multi-organ dysfunction in a significant minority of patients.
Study: Adenosine A2A Receptor (A2AR) agonists improve survival in K28-hACE2 mice following SARS-CoV-2 infection. Image Credit: CKA / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
The observation of a cytokine storm, with severe lymphopenia and intense infiltration of the lungs by mononuclear cells, as well as of the spleen, lymph nodes, and other organs, in severe and critical COVID-19 has led to intensive research on therapies to prevent and treat the progression of these events.
Among these therapeutic treatments include the repurposed antiviral remdesivir, a viral polymerase inhibitor, as well as the recently released drug Paxlovid. Paxlovid is an oral viral replication inhibitor that is currently used under emergency use authorization to treat mild to moderate COVID-19 in an effort to reduce the rate of hospitalization and death in high-risk individuals.
Dexamethasone, a synthetic glucocorticoid with powerful anti-inflammatory activity, has also been found to reduce hospital stays and mortality in patients with severe COVID-19. Certain combinations of these drugs have been associated with greater benefit.
For instance, remdesivir, while relatively ineffective by itself, confers benefit when used with dexamethasone or the Janus kinase (JAK) inhibitor baricitinib. Similarly, other molecules that target steps in the inflammatory cascade, such as the inhibition or neutralization of interferon γ (IFN- γ), interleukin-6 (IL-6) receptors, B- or T-cell ablation, T-cell modulation or blockers of the IL-1 cytokine family have also been used to treat COVID-19.
Adenosine antagonists are another potential treatment approach. In some situations, adenosine in an inhaled or aerosolized form has been used to help improve the respiration rate of patients with severe COVID-19.
Adenosine is a powerful immunosuppressive nucleoside that acts through both innate and adaptive immune responses. Moreover, this agent binds to four types of receptors, of which the A2A and A2B types are known activators of the endogenous immunosuppressive pathways.
When adenosine binds to these receptors on the immune cells, the immunosuppressive effects are initiated. This subsequently limits tissue damage and inflammation, promotes increased oxygen supply to enhance tissue repair, blocks pro-inflammatory cell recruitment and cytokine release, reduces the expression of endothelial adhesion molecules, and increases the growth of new blood vessels.
The A2A receptor (A2AR) is central in protecting the lungs from high neutrophil concentrations and increased vascular permeability, the latter of which can lead to the leakage of exudate that prevents proper gas exchange. Thus, A2AR agonists are useful in treating asthma and chronic obstructive pulmonary disease by blocking the flooding of the bronchioalveolar space with white cells and suppressing the activation of inflammatory cells, thereby reducing the extent of immunosuppression.
In lung injury caused by exposure to toxic lipopolysaccharide (LPS) antigens, A2AR activation on myeloid cells leads to reduced cytokine release, neutrophil recruitment, and adhesion to the pulmonary endothelium.
Some notable A2AR agonists include regadenoson and apadenoson. Regadenoson causes the myocardial vessels to dilate without exercise, thus helping to achieve successful perfusion imaging at rest. Comparatively, apadenoson has been shown to improve survival rates in mice with sepsis, with synergistic activity observed when administered with ceftriaxone.
Both of the ATAR agonists also prevent lung injury due to ischemia-reperfusion or transplant-related injury. For this reason, the scientists in the current study tested apadenoson in K18-hACE2 mice exposed to SARS-CoV-2.
Study findings
The use of apadenoson following exposure to SARS-CoV-2 led to better outcomes in infected mice, with up to 30% of mice surviving to the end of the 12-day post-infection period. No significant effect was observed in mice pretreated with the drug. In both groups that received the apadenoson, the illness was less severe as compared to controls.
Apadenoson was also found to induce a cytokine profile associated with reduced weight loss and a better chance of survival or recovery. Comparatively, the higher cytokine profile in the control group, though associated with slower weight loss, merely delayed the eventual death.
The viral burden in the lungs was inversely correlated with weight loss in mice treated after exposure; however, the lowest staining was observed in mice with the greatest weight loss. The lack of correlation between viral staining and weight loss indicates that lung injury and severe illness is due to inflammatory phenomena with immune dysregulation, rather than direct viral injury.
The surviving mice in both treatment groups had no detectable viral titers, unlike the controls. The viral burden was lower in the post-infection treatment group. Apadenoson may thus act by reducing the viral burden while simultaneously reducing the inflammatory response to SARS-CoV-2.
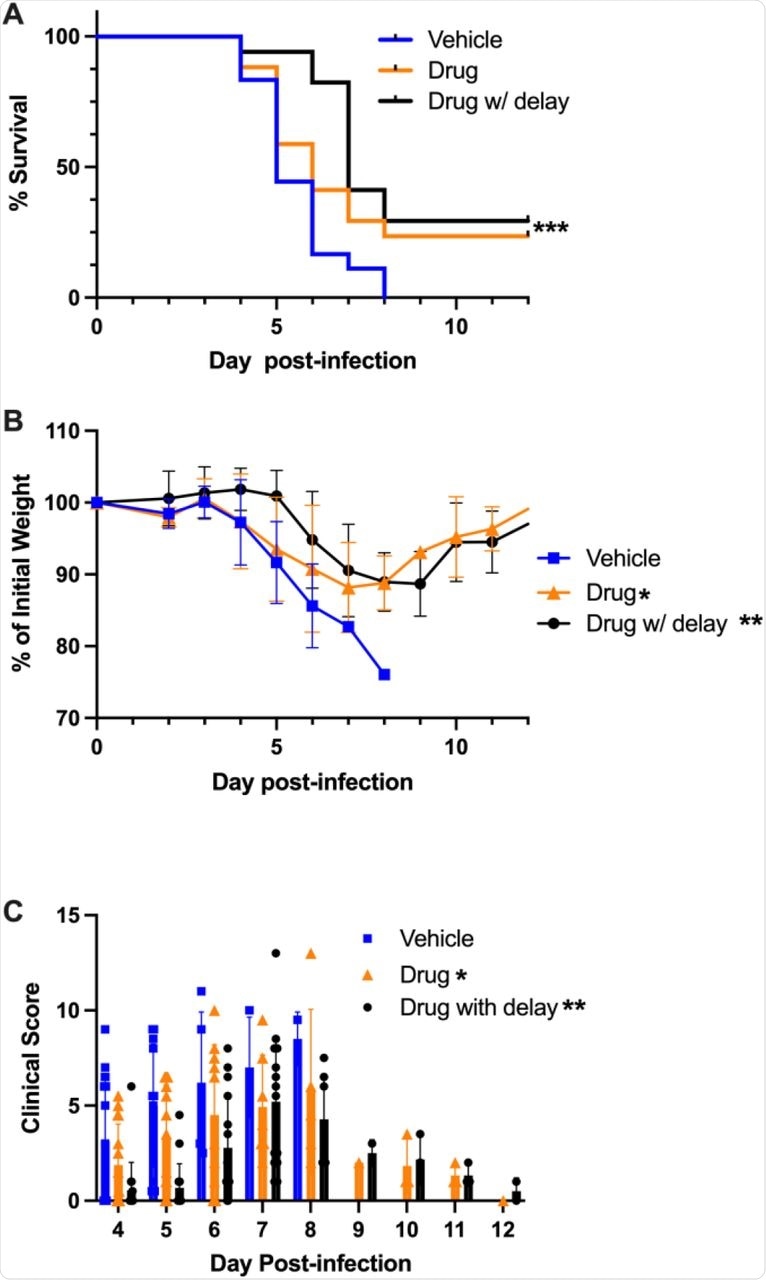 Treatment with apadenoson improved survival and delayed time to death.
Treatment with apadenoson improved survival and delayed time to death.
Implications
The findings of the current study show that the adenosine agonist apadenoson reduces the inflammatory immune response, while also reducing certain cytokines and chemokines to almost baseline levels. The nine cytokines that were higher in the control mice have been reported to be increased in SARS-CoV-2 infection, indicating its inflammatory nature.
Despite the lack of a strong association between viral burden in the lungs and weight loss, apadenoson-treated mice had lower viral titers in lung tissues, thus indicating a possible action on viral clearance.
Overall, these findings suggest that the use of anti-inflammatory drugs such as apadenoson is beneficial, and may even reduce viral burden in the lung. These data support the use and further exploration of apadenoson, or other adenosine agonists, as supportive care for COVID-19 patients.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Mann, B. J., Chhabra, P., Ma, M., et al. (2022). Adenosine A2A Receptor (A2AR) agonists improve survival in K28-hACE2 mice following SARS-CoV-2 infection. bioRxiv. doi:10.1101/2022.02.25.481997. https://www.biorxiv.org/content/10.1101/2022.02.25.481997v1.
- Peer reviewed and published scientific report.
Chhabra, Preeti, Barbara Mann, Mingyang Ma, Sanford Feldman, Joel Linden, and Kenneth Brayman. 2022. “P16.26: Adenosine A2A Receptor (A2AR) Agonists Improve Survival in K28-HACE2 Mice and Syrian Hamsters Following SARS CoV-2 Infection.” Transplantation 106 (9S): S739–39. https://doi.org/10.1097/01.tp.0000889712.84000.f6. https://journals.lww.com/transplantjournal/Citation/2022/09001/P16_26__Adenosine_A2A_Receptor__A2AR__Agonists.1132.aspx.