The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a coronavirus with a single-stranded RNA (ssRNA) genome that belongs to the Coronaviridae family which includes four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Enveloped positive ssRNA viruses are classified as Nidovirales.
The spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins are the primary structural proteins necessary for the structural stability and virulence of SARS-CoV-2. These proteins are encoded by the SARS-CoV-2 enveloped positive-sense ssRNA.
SARS-CoV-2 binds to the host cell by attaching its S protein to the angiotensin-converting enzyme 2 (ACE2) receptor through its S1 region's receptor-binding domains (RBD). In addition to the ACE2 receptor, the transmembrane serine protease 2 (TMPRSS2) also mediates viral entry into the host cell.
Study: Nucleic Acid-Based COVID-19 Therapy Targeting Cytokine Storms: Strategies to Quell the Storm. Image Credit: ktsdesign / Shutterstock.com
About the study
The use of microRNAs (miRNAs) to interfere with the expression of viral proteins provides a diverse framework for controlling SARS-CoV-2 infection. Furthermore, several studies have found that the coronavirus disease 2019 (COVID-19)-induced cytokine storm can be reduced by targeting these miRNAs.
In a recent Journal of Personalized Medicine study, researchers examine current nucleic acid-based treatments against COVID-19 and their methods of action, as well as prospective future possibilities considering the emergence of new SARS-CoV-2 variants of concern.
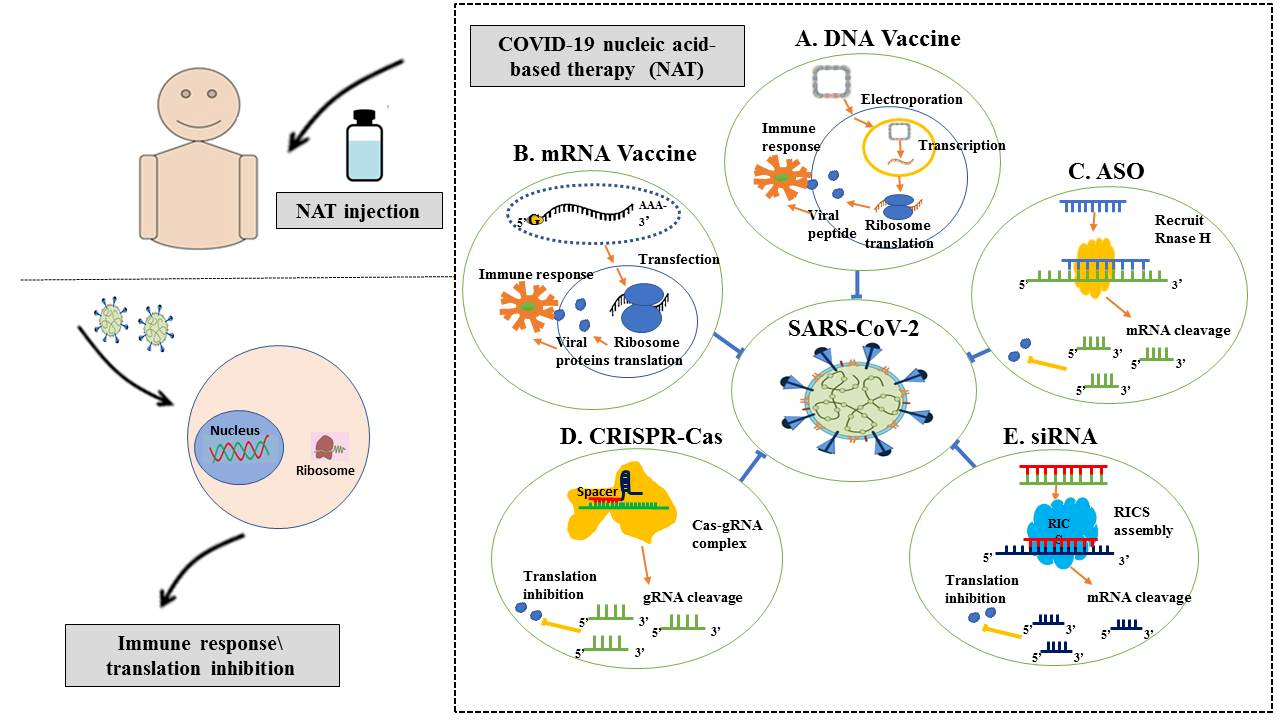
Nucleic acid-based vaccines
Nucleic acid techniques have received much interest in the realm of next-generation vaccines. In 1990, the first proof of concept for a DNA vaccine was carried out, which involved the injection of DNA or RNA molecules into a mouse model. These molecules expressed luciferase, beta-galactosidase, chloramphenicol acetyltransferase, and reporter genes in vivo, which can be identified for up to two months following infection.
Since 1990, several institutes have conducted research on vaccines, cancer immunotherapies, and immunological treatments for autoimmune and allergy conditions using plasmid DNA and messenger RNA (mRNA). Given the increased frequency of epidemics, advancements in DNA and RNA vaccine production approaches have also been crucial.
The first vaccine to receive global authorization for use against COVID-19 was the Pfizer–BioNTech BNT162b2 vaccine. The Pfizer–BioNTech BNT162b2 vaccine is a lipid nanoparticle-formulated, nucleoside-modified RNA (modRNA) that encodes the full-length SARS-CoV-2 S protein with two proline alterations to secure it in the prefusion conformation.
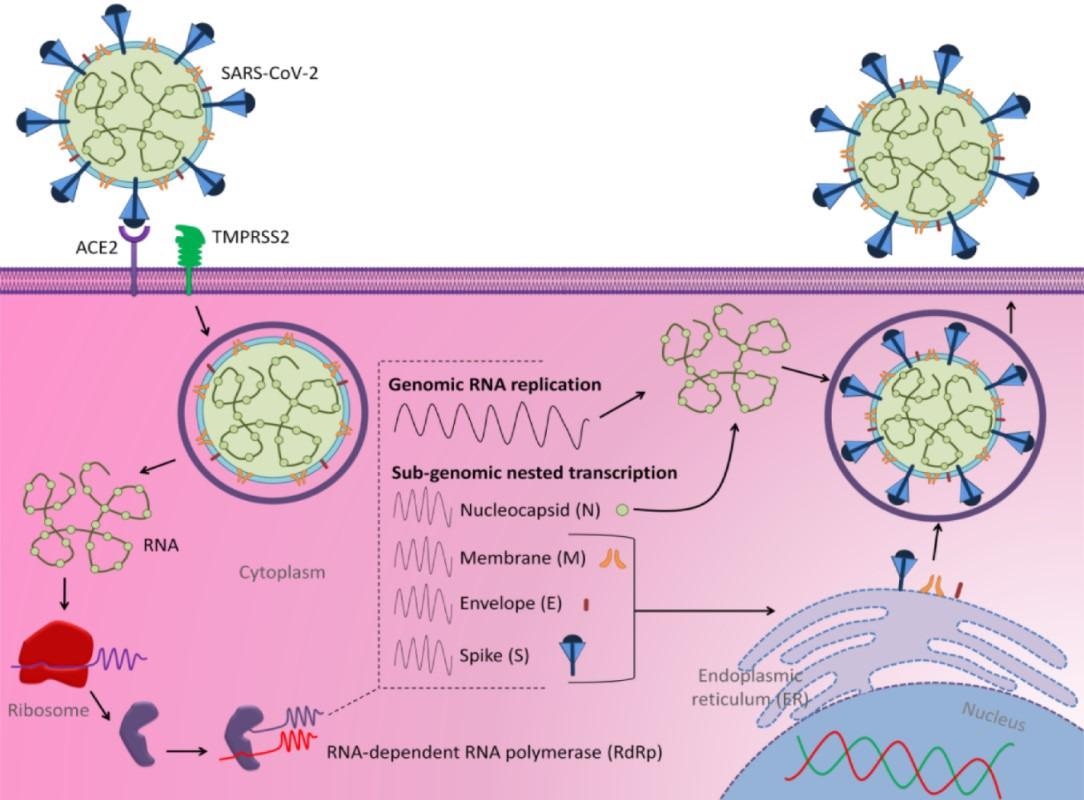
Structure scheme of SARS-CoV-2 and its mechanisms of cell entry and replication (adapted from Al-Hatamleh et al., 2020
Many vaccinations induce an immune response by introducing a weakened or inactivated pathogenic organism to the body. However, the Pfizer mRNA vaccine introduces a piece of genetic information from SARS-CoV-2 into the host cells, thus inducing an immune response. The mRNA from the vaccine is released into the host cell cytoplasm following injection, where it gets broken down and destroyed after viral proteins are formed on the cell surface.
The genetic code from the vaccine provides ‘instructions’ to the host cells to manufacture S proteins. These S proteins induce an immune response, which results in the production of neutralizing antibodies and robust interferon-γ (IFN-γ) -producing and interleukin-2 (IL-2)-producing CD8+ cytotoxic T-cells and CD4+ type 1 helper T (Th1) cell responses, with the capacity to identify and respond to any subsequent SARS-CoV-2 infection.
Nucleic acid-based therapeutics
There is considerable potential in the use of miRNAs to reduce the cytokine storm. During an active SARS-CoV-2 infection, there are multiple mechanisms that could be utilized to control a cytokine storm by miRNAs. For example, miRNAs could be used to manipulate the implicated inflammatory signaling pathways or even modulation of the inflammatory response-related host miRNAs.
It was therefore proposed that miRNA mimics could be used as cytokine storm anti-inflammatory agents by targeting the 3′UTR of pro-inflammatory mRNAs. The endothelial dysfunction and inflammatory response associated with COVID-19 were shown to be regulated by miR-26a-5p, miR-29b-3p, and miR-34a-5p. MiR-26a-5p has also been discovered to downregulate IL-6 and ICAM-1, whereas miR-29b-3p has been found to downregulate IL-4 and IL-8, all of which are low-expressed during infection. As a result, these three miRNAs, particularly miR-26a-5p, which targets IL-6 and may lower mortality, could be possible options for reducing the cytokine storm.
Implications
The vaccination of a very high percentage of the global population with COVID-19 mRNA vaccines will produce vital data on non-viral RNA delivery, immunological response, safety, and efficacy. Researchers may be able to leverage these discoveries to develop a more efficient strategy in the future by incorporating small interfering RNAs (siRNAs), miRNA mimics, antagomirs, or even genes in the same or similar lipid nanoparticles that are used in vaccines for health applications and gene therapy.
Among the therapeutic nucleic acid (TNA)-based methods, miRNAs are highly flexible and efficient options that have considerable promise in reducing the cytokine storm. Additionally, COVID-19 is linked to many miRNAs in the host or those encoded by SARS-CoV-2, some of which may even impair the immune system. As a result, the cytokine storm can be controlled by targeting these miRNAs that are directly involved in the pathogenesis of COVID-19.
Journal reference:
- Abusalah, M. A. H., Khalifa, M., Al-Hatamleh, M. A. I., et al. (2022). Nucleic Acid-Based COVID-19 Therapy Targeting Cytokine Storms: Strategies to Quell the Storm. Journal of Personalized Medicine. doi:10.3390/jpm12030386.